Antibody or antigen-binding fragment thereof that specifically recognizes b cell malignancies, chimeric antigen receptor comprising same, and uses thereof
A technology that combines fragments and antigens, applied in receptor/cell surface antigen/cell surface determinant, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, animal cells, etc. Cancer deaths and more
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
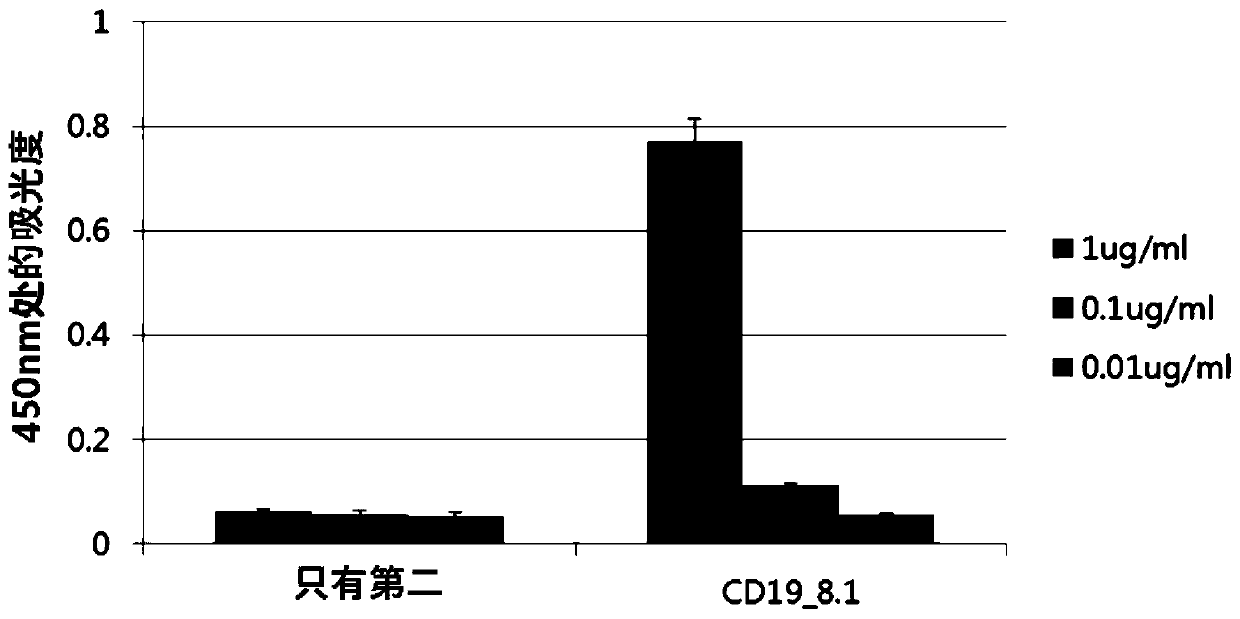
[0201] Example 1: Development of antibodies related to CD19
[0202] In order to develop antibodies, the extracellular domain (ECD, Extracellular domain) of human CD19 protein is produced in animal cells and used as an antigen. Bind the C-terminus of the extracellular structure to the hinge of human immunoglobulin G1 (IgG1) and the Fc site (CH 2 -CH 3 ) morphological deoxyribonucleic acid (DNA) cloned in pCEP4 vector (Invitrogen, USA). Subsequently, the cloned above vectors were transiently transformed into FreeStyle TM 293F (Invitrogen, USA) cells to obtain CD19-ECDFc fusion protein. Phage bio-panning was performed by using the OPLA library with the CD19-ECDFc fusion protein described above. The antibody library uses VCSM13 helper phage (helper phage) to obtain the antibody library in the form of phage and use it for panning. The number of library phages that initially bind to the antigen is 10 13 . The panning rounds (panning round) were carried out to 4 rounds, and as...
Embodiment 2
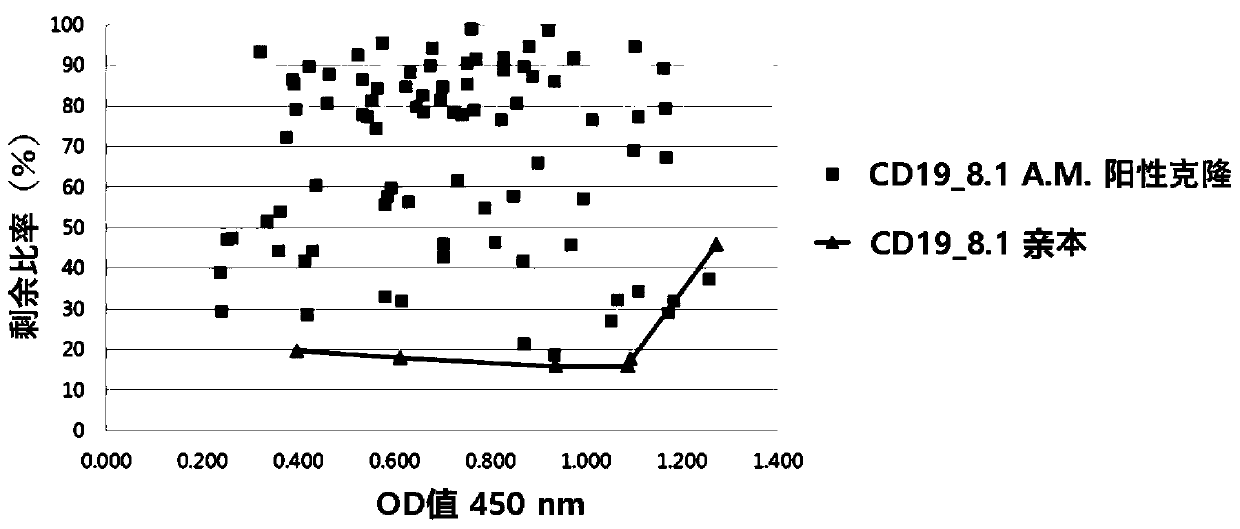
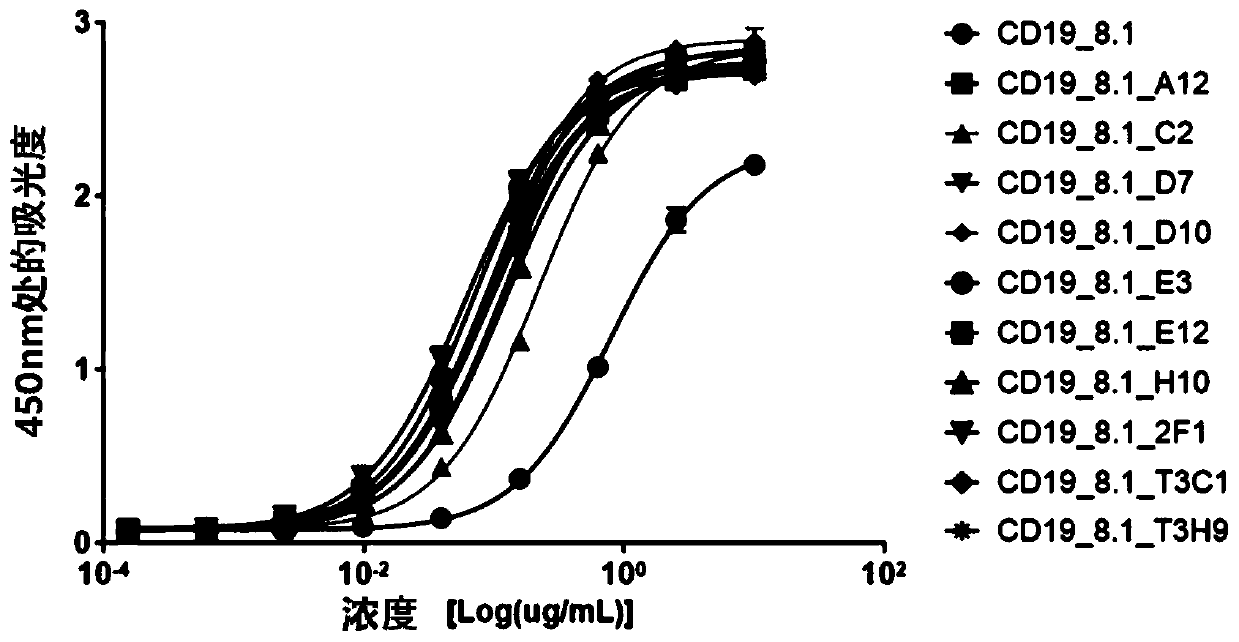
[0206] Example 2. Enhanced affinity for developed antibody fragments
[0207] In order to obtain antibody fragments that bind CD19 better than CD19_8.1, a new sub-library was prepared by combining the heavy and light chain libraries. To generate sub-libraries, oligonucleotides containing NNK degenerate codons were used. CD19_8.1 antibody fragment was used as template DNA. Random codons were introduced into five complementarity determining regions (CDRs) except HCDR3 by polymerase chain reaction (PCR). The amplified antibody fragments were purified with QIAquick Gel Extraction Kit (QIAGEN, USA). The antibody fragment and the pComb3XSS vector were cut with sfiI restriction enzyme, and after ligation, they were transfected into ER2537 to prepare a phage library. Based on the prepared phage library, antibodies were screened in the same manner as in Example 1.
[0208] In order to screen antibodies with higher affinity among the selected antibodies, an enzyme-linked immunosorbe...
Embodiment 3
[0219] Example 3: Preparation of lentiviruses comprising chimeric antigen receptors linked to developed antibody fragments
[0220]A chimeric antigen receptor was developed using the developed antibody CD19_8.1. After codon-optimizing the CD8 leader sequence, CD19_8.1 in the form of single-chain variable region (scFv), CD8 hinge region, CD137 transmembrane region, and CD3ζ cytoplasmic region, the pLenti6-V5 / DEST was slowed down by SpeI / PacI Viral vectors (Invitrogen, USA) cut and ligated chimeric antigen receptors. The prepared construct (sequence No. 181 in the sequence listing) was confirmed by base sequence analysis.
[0221] The prepared lentiviral construct was transduced into the Lenti-X 293T (Takara BioInc, Japan) cell line together with pCMV-dR8.91, which is a vesicle containing a protein encoding as a viral capsid protein. Stomatitis Indiana virus G protein (VSV-G, vesicular stomatitis indiana virus G protein) nucleic acid and gag, pol and rev gene plasmids. Transd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com