Compound 3, 7, 11-cembratriene-2, 6-diol as well as preparation method and application thereof
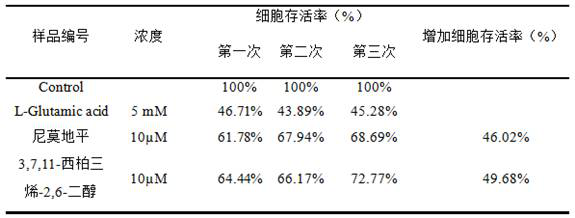
A technology of cerebrotriene and compound, which is applied to compound 3,7,11-cerebrotriene-2,6-diol and its preparation and application fields, and can solve the problem of unseen patent documents of preparation methods, unseen nerve cells, etc. It can achieve good neuroprotective effect, prevent and treat neurodegenerative diseases, and inhibit neuronal cell damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
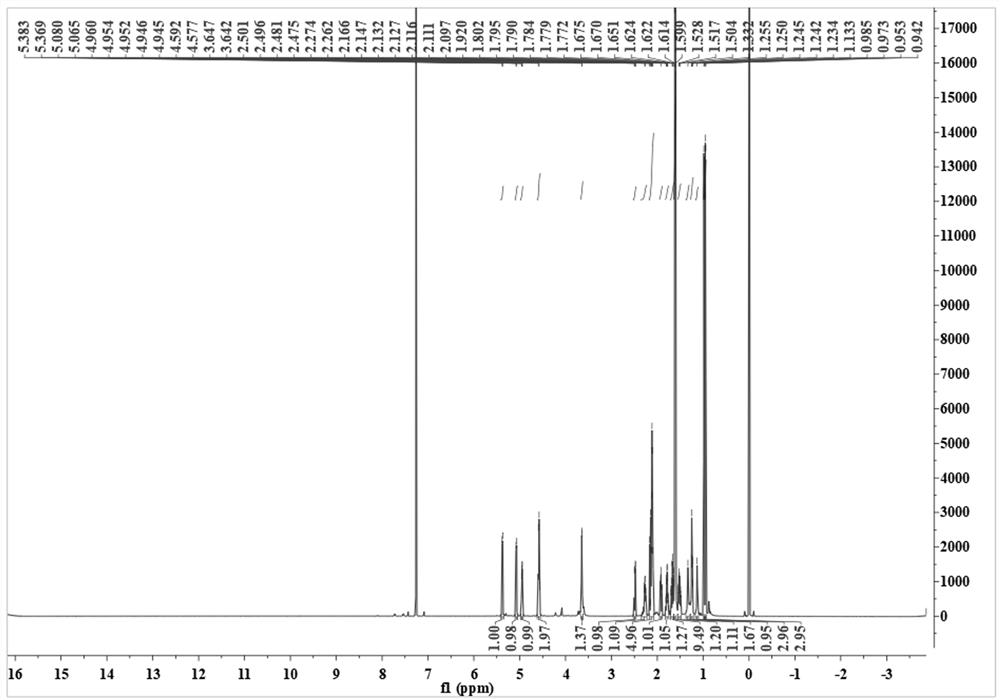
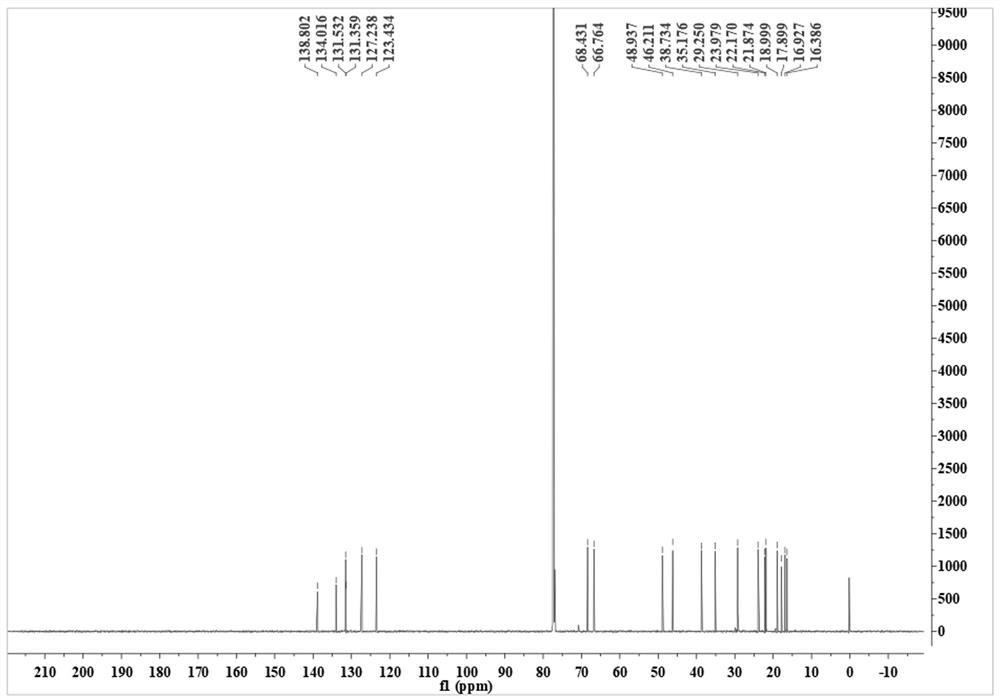
[0039] The preparation method of compound 3,7,11-cembratriene-2,6-diol, the steps are as follows:
[0040] 1) 5 kg of fresh tobacco leaves were rinsed three times with dichloromethane and filtered. At 45 °C, dichloromethane was distilled and concentrated under reduced pressure to obtain 500 g of brown extract. The extract was redissolved in 1 L of dichloromethane, and washed with 500 mL of 5% phosphoric acid solution. After washing three times, the dichloromethane phase was collected and distilled under reduced pressure to obtain 386 g of a crude extract containing hucipressanes.
[0041] 2) Take 80 g of the crude extract and separate it by silica gel column chromatography, first use petroleum ether / ethyl acetate with volume ratios of 10:1, 8:1, 6:1, 4:1, 1:1, 3:4 The eluate was eluted with ethyl acetate and methanol in sequence, collected once in 250 mL, and the crude product of the new compound 3,7,11-cembratriene-2,6-diol was obtained after detection and collection.
[00...
Embodiment 2
[0049] The preparation method of compound 3,7,11-cembratriene-2,6-diol, the steps are as follows:
[0050] 1) 5 kg of fresh tobacco leaves were rinsed three times with dichloromethane and filtered. At 43°C, dichloromethane was distilled and concentrated under reduced pressure to obtain 500 g of brown extract. The extract was redissolved in 0.9 L of dichloromethane, and washed with 550 mL of 8% phosphoric acid solution. After washing three times, the dichloromethane phase was collected and distilled under reduced pressure to obtain 377 g of a crude extract containing hucipressanes.
[0051] 2) Take 80 g of the crude extract and separate it by silica gel column chromatography, first use petroleum ether / ethyl acetate with volume ratios of 10:1, 8:1, 6:1, 4:1, 1:1, 3:4 The eluate was eluted with ethyl acetate and methanol in sequence, collected once in 250 mL, and the crude product of the new compound 3,7,11-cembratriene-2,6-diol was obtained after detection and collection.
[0...
Embodiment 3
[0057] The preparation method of compound 3,7,11-cembratriene-2,6-diol, the steps are as follows:
[0058] 1) 5 kg of fresh tobacco leaves were rinsed three times with dichloromethane and filtered. At 45 °C, dichloromethane was distilled and concentrated under reduced pressure to obtain 500 g of brown extract. The extract was redissolved in 0.8 L of dichloromethane, and washed with 60 mL of 7% phosphoric acid solution. After washing three times, the dichloromethane phase was collected and distilled under reduced pressure to obtain 390 g of a crude extract containing hucipressanes.
[0059] 2) Take 80 g of the crude extract and separate it by silica gel column chromatography, first use petroleum ether / ethyl acetate with volume ratios of 10:1, 8:1, 6:1, 4:1, 1:1, 3:4 The eluate was eluted with ethyl acetate and methanol in sequence, collected once in 250 mL, and the crude product of the new compound 3,7,11-cembratriene-2,6-diol was obtained after detection and collection.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com