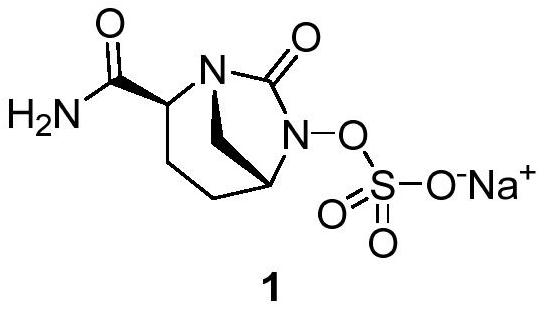
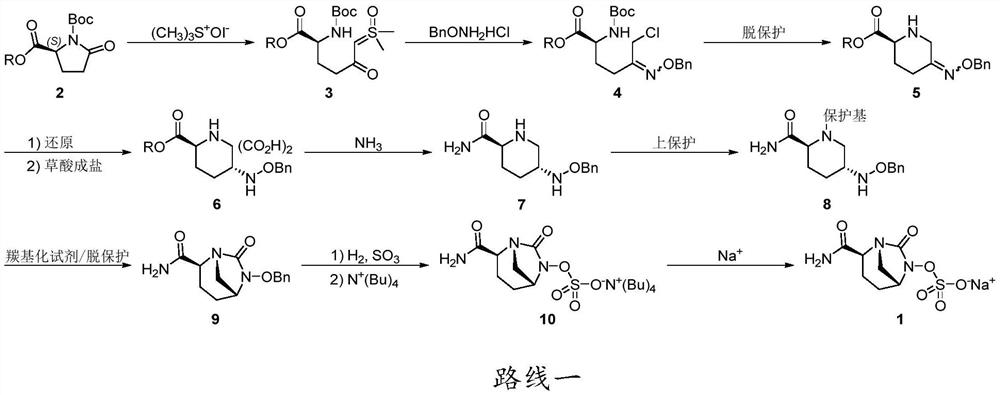
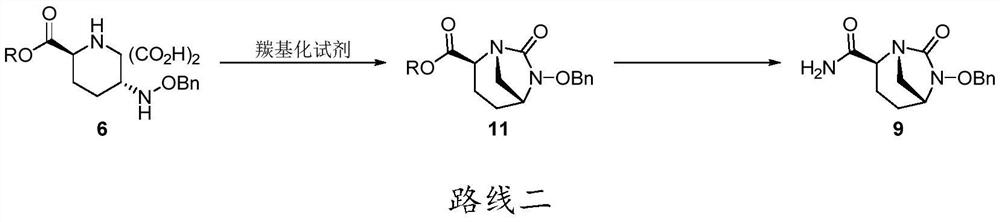
A kind of preparation method of Avibactam sodium intermediate
A technology of avibactam sodium and intermediates, applied in the field of medicinal chemistry, can solve the problems of long route, high cost, low atom utilization rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Embodiment 1: the preparation of compound (I)
[0136] Compound (I) was prepared according to the method in the example of patent document CN105061425B.
Embodiment 2
[0137] Embodiment 2: the preparation of compound (V) oxalate
[0138] Step (1): Preparation of compound (II)
[0139] Trimethylsulfoxide iodide (77.4g, 352mmol, 1.0eq) and potassium tert-butoxide (39.5g, 352mmol, 1.0eq) were sequentially added to a solution of dimethylsulfoxide (500mL) at 10-15°C , and the mixture was stirred for 1-1.5 hours. The mixture was slowly added to a solution of compound (I) (100.0 g, 352 mmol, 1.0 eq) in dimethyl sulfoxide (500 mL) at 20-25° C., and the mixture was stirred at 20-25° C. for 1-2 hours until The HPLC method monitored the reaction until completion. The reaction was quenched by the addition of saturated aqueous ammonium chloride (900 mL). The product was extracted 3-4 times with ethyl acetate (1800 mL), and the resulting organic solution was washed with saturated brine. The organic solution was concentrated under vacuum to a final volume of 1000 mL to obtain a solution containing compound (II), which was directly carried out to th...
Embodiment 3
[0146] Embodiment 3: the preparation of compound (V) oxalate
[0147] Step (1): Preparation of compound (II)
[0148] Trimethylsulfoxide iodide (154.8g, 703mmol, 2.0eq) and sodium hydride (60%, 28.1g, 703mmol, 2.0eq) were sequentially added to a solution of dimethylsulfoxide (500mL) at 10-15°C , the mixture was stirred for 1-1.5 hours. The mixture was slowly added to a solution of compound (I) (100.0 g, 352 mmol, 1.0 eq) in dimethyl sulfoxide (500 mL) at 20-25° C., and the mixture was stirred at 20-25° C. for 1-2 hours until The HPLC method monitored the reaction until completion. The reaction was quenched by the addition of saturated aqueous ammonium chloride (900 mL). The product was extracted 3-4 times with isopropyl acetate (1800 mL), and the resulting organic solution was washed with saturated brine. The organic solution was concentrated under vacuum to a final volume of 1000 mL to obtain a solution containing compound (II), which was directly carried out to the n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com