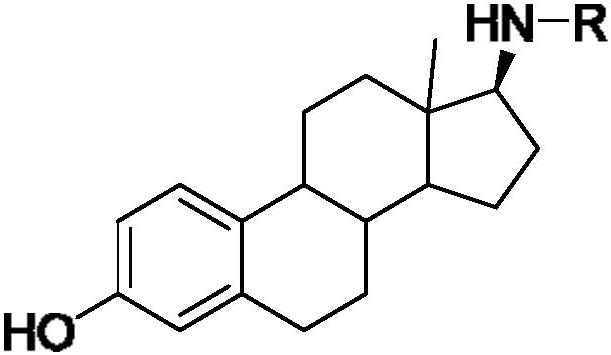
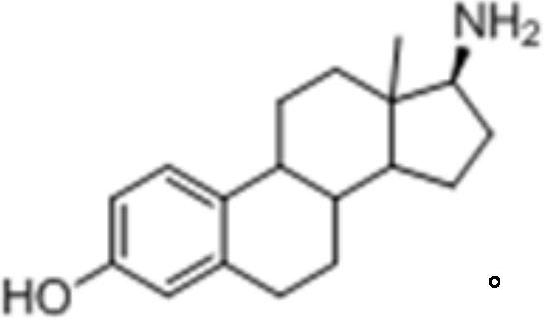
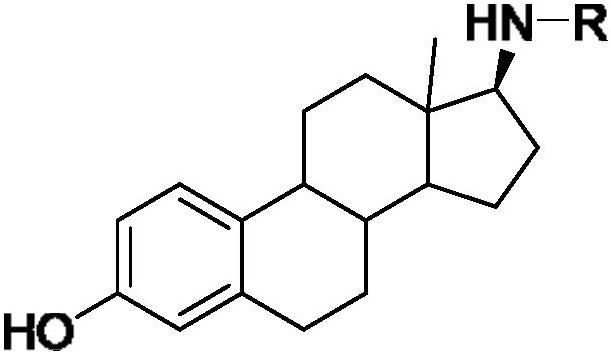
17-amide estrogen compound and its preparation method and application
A compound, estrogen technology, applied in the field of 17-amide estrogen compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of N-((17β)-3-hydroxyl-1,3,5(10)-estratrien-17-yl)-o-methoxybenzamide:
[0045] Take 150 mg of the second compound 2, dissolve it in 10 mL of dichloromethane, add 1 mL of triethylamine, slowly add 90 μL of o-methoxybenzoyl chloride, follow the reaction by TLC, react at room temperature for 3 h, spin dry under reduced pressure, and extract with ethyl acetate three times , washed 2 times with water, washed 1 time with saturated brine, dried over anhydrous sodium sulfate, spin-dried, separated by column chromatography (eluent is V 二氯甲烷 :V 甲醇 =100:1), a white solid was obtained with a yield of 20%, m.p.235~236°C, and the structure of the product was determined to be N-((17β)-3-hydroxyl-1,3,5(10 )-estratrien-17-yl)-o-methoxybenzamide, its chemical structure is as shown in the following formula:
[0046]
[0047] Among them, R=R 1 =
Embodiment 2
[0049] Synthesis of N-((17β)-3-hydroxyl-1,3,5(10)-estratrien-17-yl)-p-toluamide:
[0050] Take 2150 mg of the second compound, dissolve it in 10 mL of dichloromethane, add 1 mL of triethylamine, slowly add 66 μL of p-toluoyl chloride, follow the reaction by TLC, react at room temperature for 3 h, spin dry under reduced pressure, extract with ethyl acetate three times, wash with water 2 times, washed 1 time with saturated brine, dried over anhydrous sodium sulfate, spin-dried, and separated by column chromatography (eluent was V 二氯甲烷 :V 甲醇 =100:1), a white solid was obtained with a yield of 20%, m.p.139~141° C., and the structure of the product was determined to be N-((17β)-3-hydroxyl-1,3,5(10 )-estratrien-17-yl)-p-toluamide, its chemical structure is as shown in the following formula:
[0051]
[0052] Among them, R=R 2 =
Embodiment 3
[0054] Synthesis of N-((17β)-3-hydroxy-1,3,5(10)-estratrien-17-yl)-3-phenyl-propionamide:
[0055] Take 150 mg of the second compound 2, dissolve it in 10 mL of dichloromethane, add 1 mL of triethylamine, slowly add 74 uL of 3-phenylpropionyl chloride, follow the reaction by TLC, react at room temperature for 3 h, spin dry under reduced pressure, and extract with ethyl acetate three times. Wash twice with water and once with saturated saline. Dry over anhydrous sodium sulfate, spin dry, and separate by column chromatography (eluent is V 二氯甲烷 :V 甲醇 =100:1), a white solid was obtained. m.p.179-181°C, the structure of the product was determined to be N-((17β)-3-hydroxyl-1,3,5(10)-estratrien-17-yl)-3-benzene by IR, NMR and MS analysis Base-propionamide, its chemical structure is as shown in the following formula:
[0056]
[0057] Among them, R=R 3 =
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com