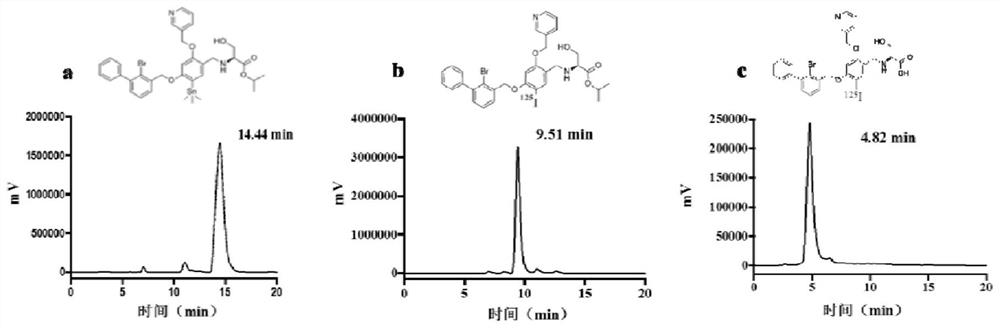
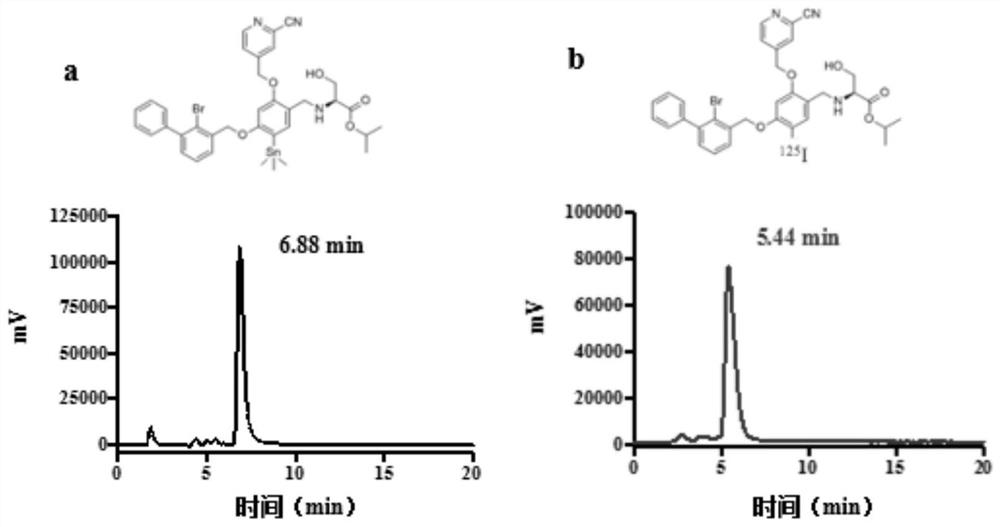
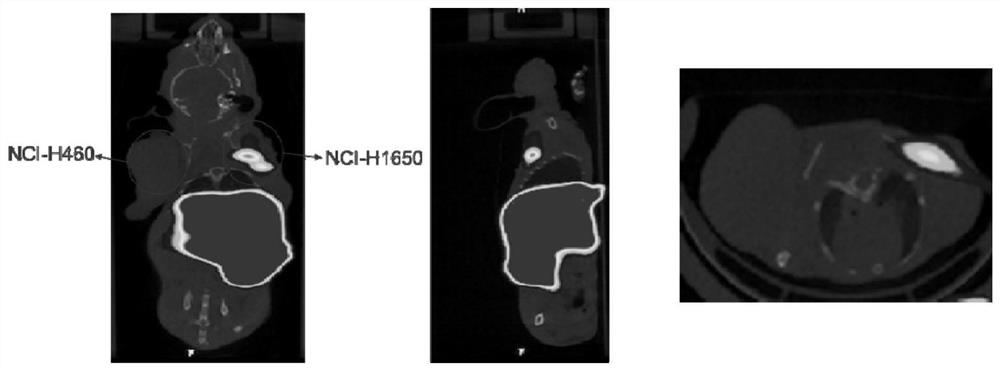
Iodine isotope labeled benzyl phenyl ether derivatives, preparation method, pharmaceutical composition and application thereof
A kind of technology of benzyl phenyl ether and derivatives, applied in the field of iodine radioisotope labelling of benzyl phenyl ether derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0273] Example 1: (S)-N-(2-(pyridine-3-methoxy)-4-(2-bromo-3-phenylbenzyloxy)-5-iodobenzyl)serine isopropyl ester
[0274]
[0275] 2-(pyridine-3-methoxy)-4-(2-bromo-3-phenylbenzyloxy)-5-iodobenzaldehyde:
[0276] Weigh 2-hydroxy-4-(2-bromo-3-phenylbenzyloxy)-5-iodobenzaldehyde (720mg, 1.414mmol) in a 50ml single-necked bottle, dissolve it completely with 25ml DMF, add cesium carbonate (1.38 g, 4.24mmol), stirred at room temperature for 10min, added 3-chloromethylpyridine hydrochloride (348mg, 2.12mmol), raised the temperature to 76°C, and reacted for 3.5h, (TLC detection, the raw material reacted completely), added water and acetic acid Extracted three times with ethyl ester, combined the organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, and separated by silica gel column chromatography to obtain 830 mg of off-white solid. 1 H NMR (400MHz, DMSO-d6)δ10.09(s,1H,-CHO),8.70(s,1H,-ArH),8.53(d,J=6.2Hz,1H,-ArH),8.02(s, 1H,-ArH),7.93(m,1H,-ArH),7....
Embodiment 2
[0279]Example 2: (S)-N-(2-(pyridine-3-methoxy)-4-(2-bromo-3-phenylbenzyloxy)-5-iodobenzyl)serine
[0280]
[0281] Weigh (S)-N-(2-(pyridine-3-methoxy)-4-(2-bromo-3-phenylbenzyloxy)-5-iodobenzyl)serine isopropyl ester (186mg, 0.254mmol), dissolved in 6ml MeOH / H 2 O (5:1) mixture, stirred at room temperature, added lithium hydroxide monohydrate (43mg, 1.018mmol), reacted at room temperature for 2h, concentrated under reduced pressure, added 1.5ml of distilled water, in ice bath conditions, with glacial acetic acid The pH was adjusted to 6, and a solid was precipitated, left to stand, and filtered with suction to obtain 128 mg of an off-white solid. 1 H NMR (400MHz, DMSO-d 6 )δ8.69(s,1H,-ArH),8.51(d,J=4.0Hz,1H,-ArH),7.94(d,J=7.8Hz,1H,-ArH),7.78(s,1H,- ArH),7.68(d,J=9.0Hz,1H,-ArH),7.51-7.45(m,1H,-ArH),7.45–7.41(m,2H,-ArH),7.41–7.32(m,5H, -ArH),6.96(s,1H,-ArH),5.30-5.20(m,4H,-2CH 2 -),3.91(s,2H,-CH 2 -),3.70-3.55(m,2H,-CH 2 -),3.15(t,J=4.9Hz,1H,-CH-).MS: [M+H] + :689.
Embodiment 3
[0282] Example 3: (S)-N-(2-(2-cyanopyridine-4-methoxy)-4-(2-bromo-3-phenylbenzyloxy)-5-iodobenzyl)serine Isopropyl ester
[0283]
[0284] 2-(2-cyanopyridine-4-methoxy)-4-(2-bromo-3-phenylbenzyloxy)-5-iodobenzaldehyde
[0285] Weigh 2-hydroxy-4-(2-bromo-3-phenylbenzyloxy)-5-iodobenzaldehyde (731mg, 1.44mmol) in a 50ml single-necked bottle, dissolve it completely with 8ml DMF, add potassium carbonate (597mg , 4.32mmol), after stirring at room temperature for 10min, add 3ml of DMF solution of 4-bromomethyl-2cyanopyridine (425mg, 2.16mmol), after reacting at room temperature for 12h, TLC detection, the raw material reaction is complete, add appropriate amount of water, precipitate a large amount of The white solid was suction filtered, washed with water, and dried to obtain 678 mg of white solid. 1 H NMR (500MHz, DMSO-d 6 )δ10.26(s,1H,-CHO),8.78(d,J=5.0Hz,1H,-ArH),8.22(s,1H,-ArH),8.10(s,1H,-ArH),7.88( d,J=5.1Hz,1H,-ArH),7.73(d,J=7.6Hz,1H,-ArH),7.56-7.46(m,3H,-ArH),7.46–7.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com