Monoclonal antibody neo-201 for the treatment of human carcinomas
A NEO-201, antibody technology, applied in the field of monoclonal antibody NEO-201 for the treatment of human cancer, can solve problems such as side effects, patients with incurable advanced diseases, recurrence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
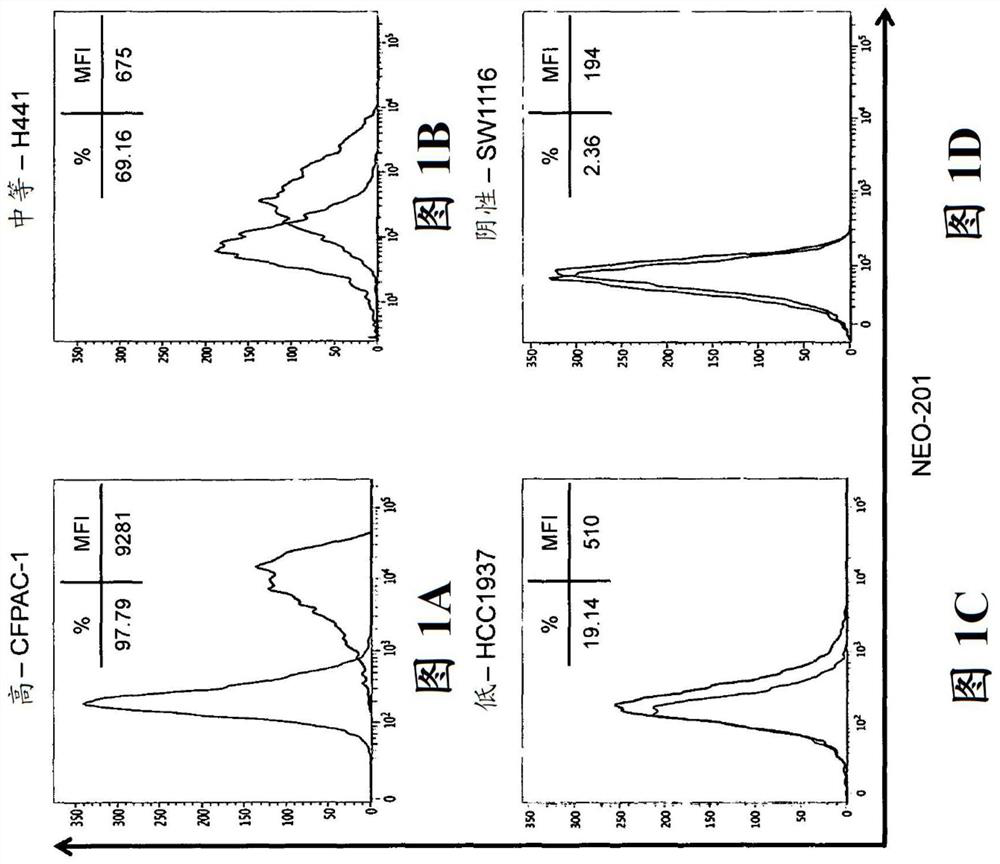
[0139] NEO-201 binds to various human cancer cell lines
[0140] Flow cytometry analysis was used to profile NEO-201 binding on a panel of human cancer cell lines. Staining profiles are summarized in Table 1, and representative histograms from cell lines with high, medium, low and negative staining are in Figures 1A-1Cshown in . Evaluation of the binding activity of NEO-201 revealed that 3 / 6 (50%) colon cancer cell lines and 4 / 5 (80%) pancreatic cancer cell lines were highly positive. When profiling non-small cell lung cancer (NSCLC) cell lines of various histological subtypes, it was determined that 3 / 5 (60%) of the adenocarcinoma cell lines responded to NEO-201, while only 1 / 4 (25 %) squamous cell carcinoma cell lines were found positive. Screening of breast cancer cell lines was also performed. Among cell lines expressing estrogen receptor (ER) or progesterone receptor (PR), 2 / 4 (50%) stained positive for NEO-201, either alone or in combination with HER2. Among HER2+ ...
Embodiment 2
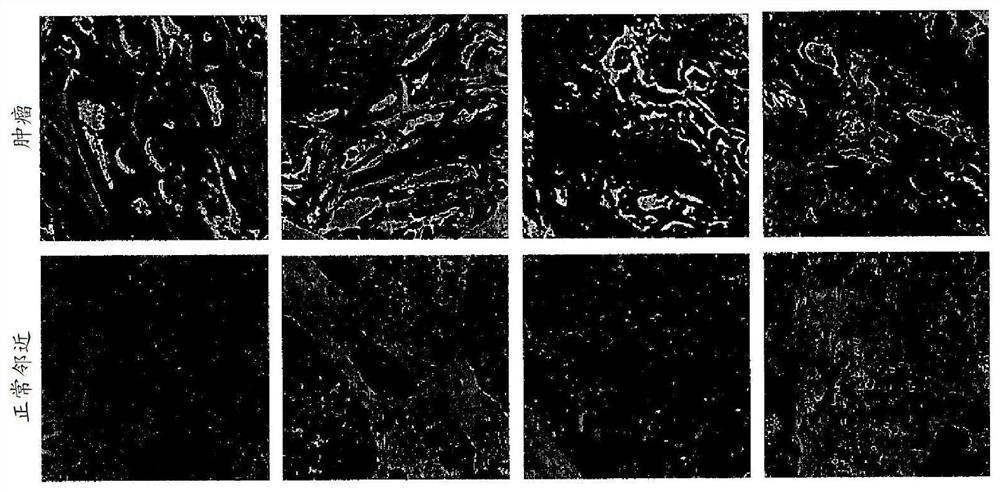
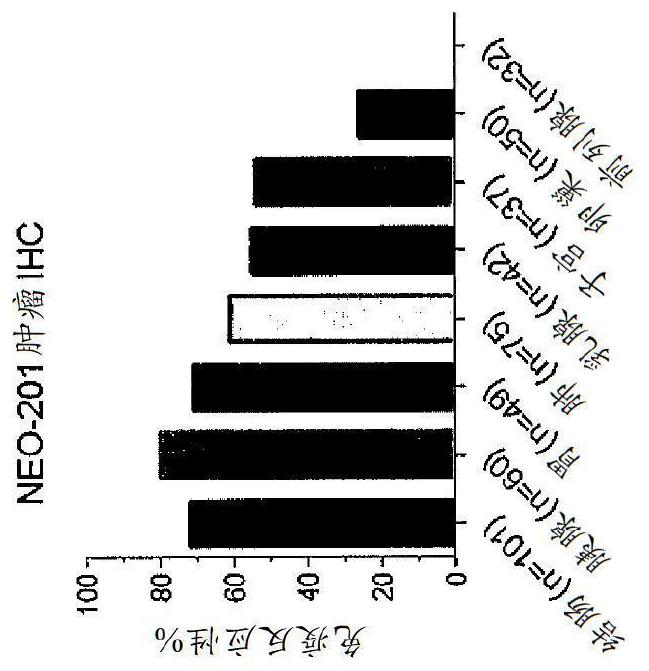
[0142] NEO-201 tissue staining is highly tumor-specific
[0143] NEO-201 reactivity from human tumor samples was investigated using immunohistochemical studies using tissue microarrays representing many samples of each cancer type. Such as Figure 2A As shown, immunoreactivity 7,829,678 with NEO-201 was completely absent in normal colon, pancreas, and lung tissues, but was highly positive in tumor tissues from these organs. Strikingly, staining was found only on tumor cells, as surrounding stromal cells were not stained ( Figure 2A ). IHC staining of microarray samples identified NEO-201 against colon cancer (72%), pancreatic cancer (80%), gastric cancer (71%), lung cancer (61%), breast cancer (55%) and uterine cancer (54%) Highly reactive. In addition, a considerable minority of ovarian cancer (26%) samples also showed positive staining, but in prostate cancer tissues ( Figure 2B ) No staining was observed. Overall, 258 / 345 (74.7%) of the sampled tumor tissues stained...
Embodiment 3
[0145] NEO-201 mediates ADCC and CDC to kill tumor cells
[0146] As a humanized IgG1 antibody, NEO-201 can theoretically mediate ADCC to kill tumor cells expressing NEO-201 antigen. To investigate this potential mechanism of action, a cell line that stained highly positive for NEO-201 was subjected to an ADCC assay utilizing human natural killer (NK) cells isolated from PBMCs from two different healthy donors (CFPAC-1 and ASPC-1). It was observed that treatment with NEO-201 enhanced the killing of CFPAC-1 and ASPC-1 to a level that was 2- to 6-fold greater than that of control IgG1-treated tumor cells ( Figure 3A ). Titration experiments were also performed and revealed that NEO-201 retained a significant ability to induce ADCC at doses as low as 0.1 μg / mL ( Figure 3B ).
[0147] CDC is a complex cascade of proteolytic cleavage culminating in activation of the membrane attack complex, which lyses antibody-bound target cells. Certain human IgG1 antibodies are capable of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com