Primer and test kit for detecting serum exosome CTD-2376I4-1 and application of primer in diagnosis and treatment of gastric cancer with liver metastasis
A technology of exosomes and kits, applied in the field of molecular diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
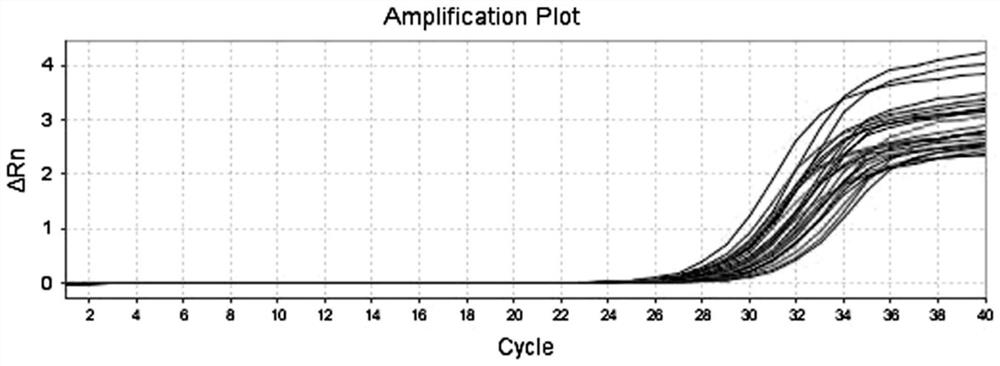
[0034] Example 1 Detection of CTD-2376I4-1 in serum exosomes
[0035] 1. Obtain the serum sample to be tested:
[0036] Serum samples from 120 cases of gastric cancer and 120 normal persons.
[0037] The samples are the serum samples of the patients with gastric cancer and normal people with coagulant added.
[0038] 2. Extract exosomes from serum samples to be tested and verify
[0039] According to the instructions of the exosome extraction kit, the exosomes in the gastric serum were extracted, and the steps were as follows: (1) Whole blood was centrifuged at 3000×g for 15 min (centrifugal radius 10 cm) to remove cells or cell debris. (2) Put the upper liquid into a centrifuge tube, add 63 μl ExoQuick reagent to 250 μl serum, put it at 4°C for 30 minutes; (3) Centrifuge the mixture at 1500×g (centrifugal radius 10cm) for 30 minutes at 4°C; ( 4) Aspirate all the supernatant, centrifuge at 1500×g (centrifugal radius 10cm) for 5 min at 4°C, and aspirate the entire supernatan...
Embodiment 2
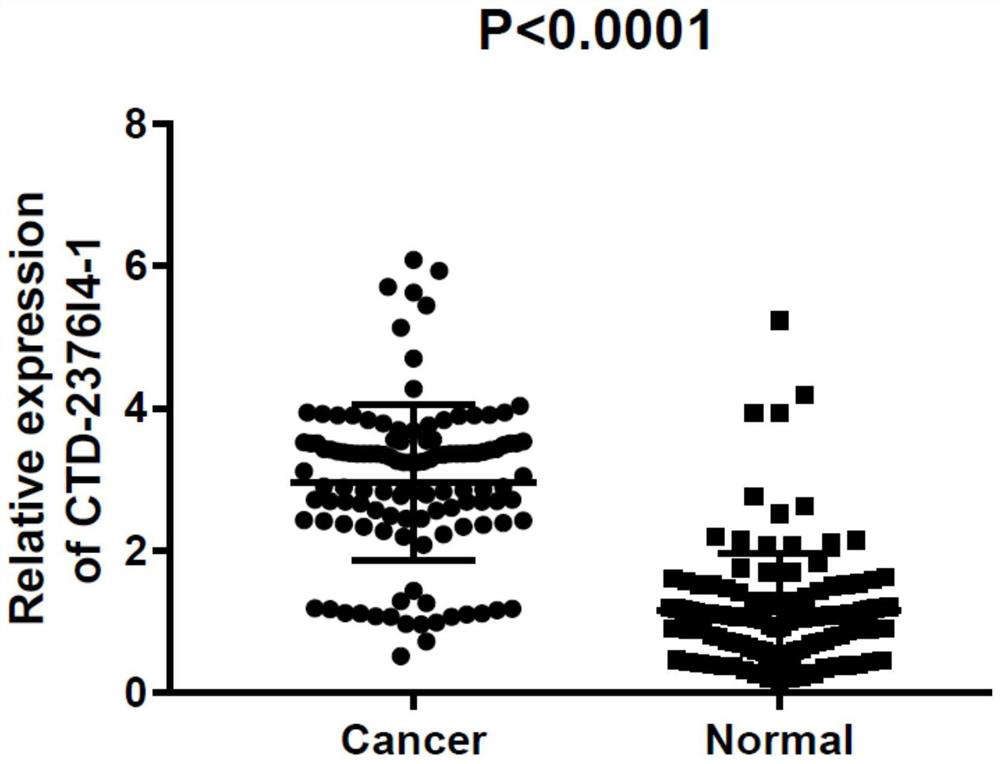
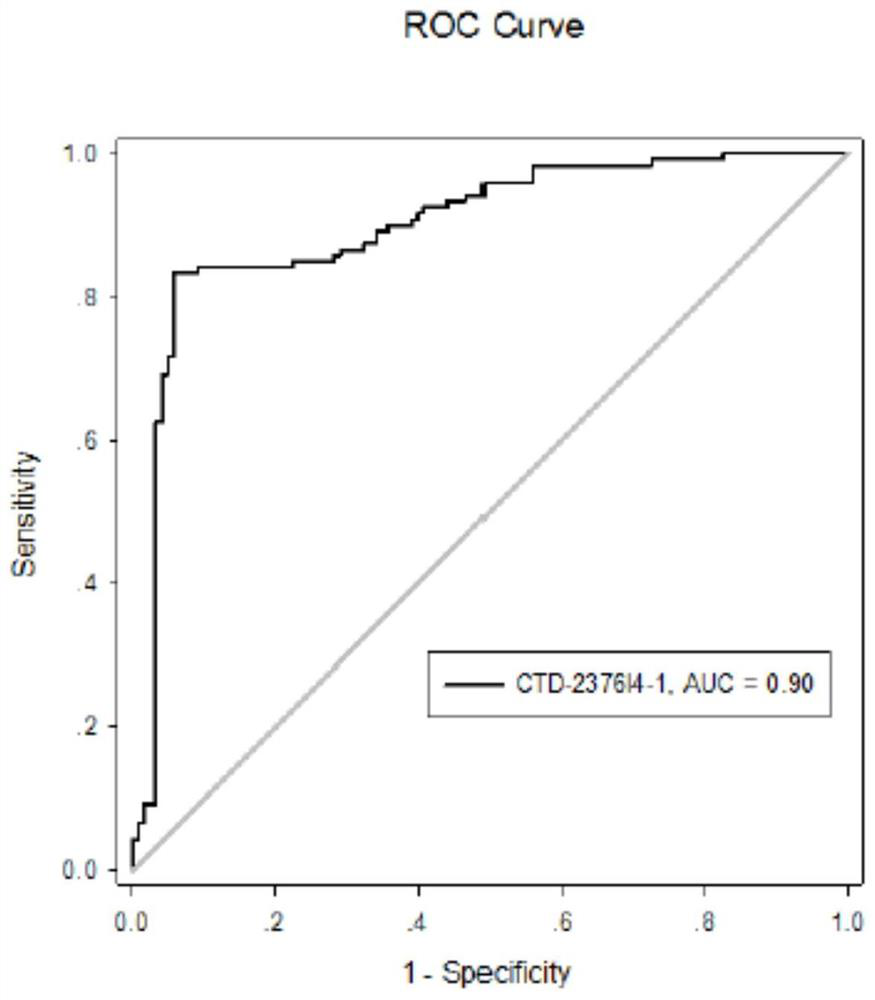
[0070] Example 2 Clinical significance of serum exosome CTD-2376I4-1
[0071] The correlation between serum exosomal CTD-2376I4-1 expression and clinical parameters such as gender, age, tumor diameter, clinical stage, degree of tissue differentiation, lymph node metastasis, and liver metastasis of gastric cancer was further analyzed. The results found that, as shown in Table 4 and Table 5, the expression level of serum exosomal CTD-2376I4-1 was significantly correlated with the depth of invasion, lymph node metastasis, liver metastasis, and clinical stage of patients (P values were 0.047, 0.036, 0.001, and 0.003, respectively. ), but not significantly correlated with gender, age, tumor location, tumor diameter, tissue differentiation, Helicobacter pylori infection and CEA level. The results of multivariate analysis suggested that the high expression of serum exosomal CTD-2376I4-1 was one of the independent prognostic factors for gastric cancer liver metastasis.
[0072] Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com