Covered stent post-release device and covered stent implantation system
A covered stent and post-release technology, which is applied in the field of medical devices, can solve the problems of low surgical success rate, high surgical difficulty, and scratched blood vessels, and achieve the effects of improving surgical efficiency, simple overall structure, and reducing scratch damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
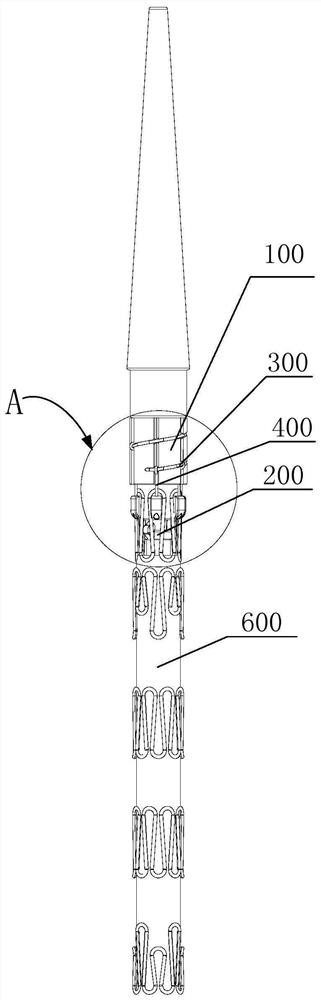
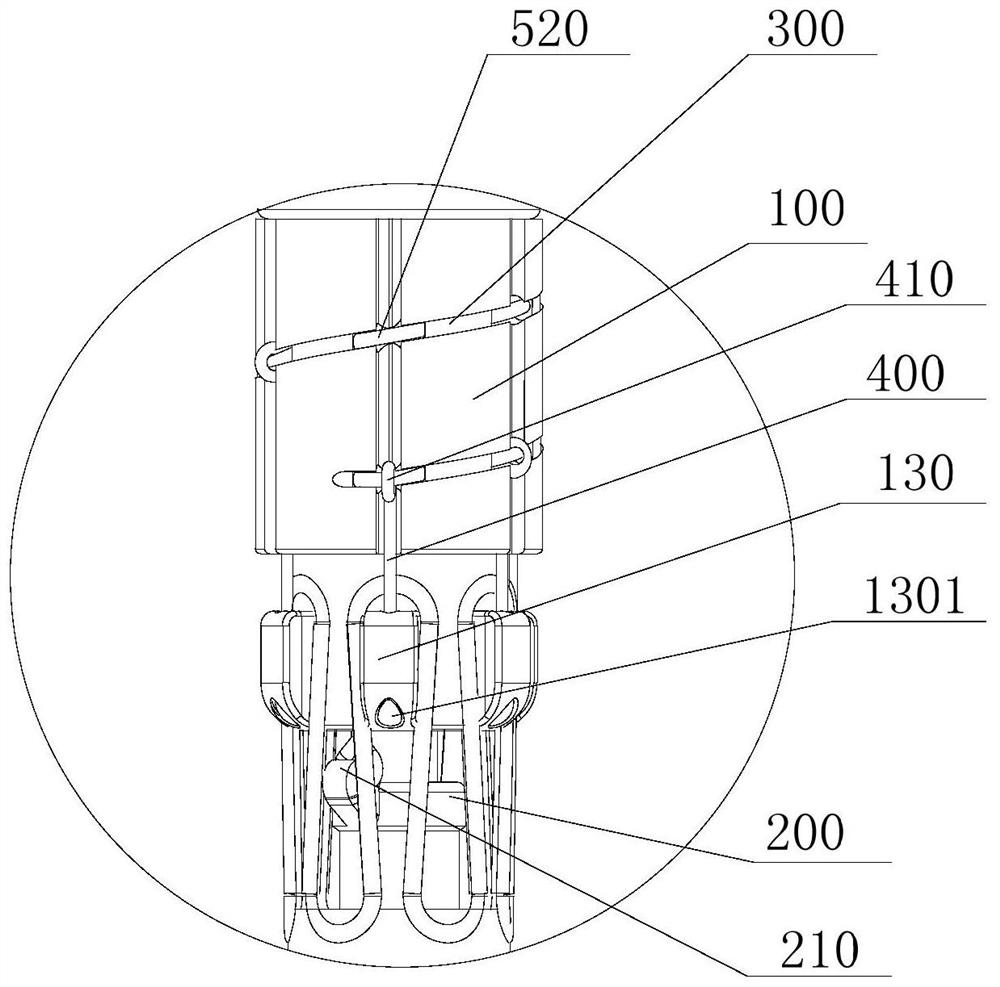
[0063] This embodiment provides a post-release device for a covered stent, refer to Figure 1 to Figure 3 , the stent-graft posterior release device includes a guide seat 100, a pull wire seat 200, a first pull wire 300, a second pull wire 400 and a locking structure.
[0064] Wherein: the first pull wire 300 and the second pull wire 400 are both made of flexible materials; the direction extending from the proximal end of the guide base 100 toward the distal end of the guide base 100 is the axial direction of the guide base 100, and the direction of the self-pull wire base 200 The direction in which the proximal end extends to the distal end of the pull wire seat 200 is the axial direction of the pull wire seat 200 . A second installation through hole 201 , which penetrates through the pull wire seat 200 along the axial direction of the pull wire seat 200 , is disposed thereon.
[0065] The first pull wire 300 is coiled around the outer peripheral surface of the guide base 10...
Embodiment 2
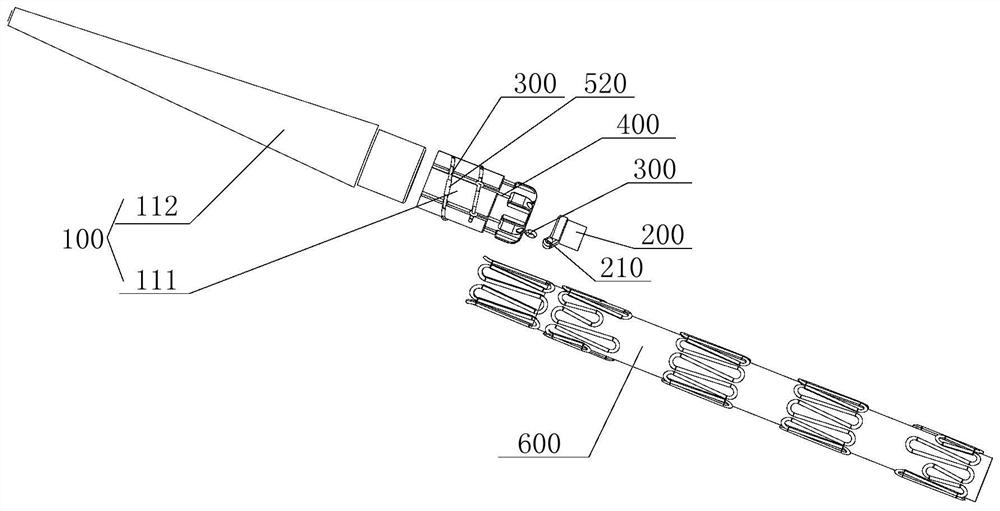
[0086] This embodiment provides a stent graft implantation system, refer to Figure 10 , Figure 11 , combined with Figure 1 to Figure 9 as well as Figure 12 , Figure 13 , the stent-graft implantation system includes an operating handle, a guide wire tube 710, an inner tube 720, an outer tube 730, and the stent-graft post-release device provided in any optional implementation in Example 1; wherein: the guide wire tube 710 Through the inner tube 720, and the guide wire tube 710 passes through the second installation through hole 201, the proximal end of the guide wire tube 710 is fixed inside the first installation through hole 101; Distal end: the distal end of the outer tube 730 and the inner tube 720 are both connected to an operating handle, and the operating handle is configured to drive the outer tube 730 and the inner tube 720 to move forward and backward.
[0087] Since the stent-graft implantation system provided in this embodiment includes the stent-graft post-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com