Quinoline derivatives for the treatment of triple negative breast cancer
A triple-negative breast cancer, post-treatment technology, applied in the field of medicine, can solve the problem of lack of necessity and urgency of treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
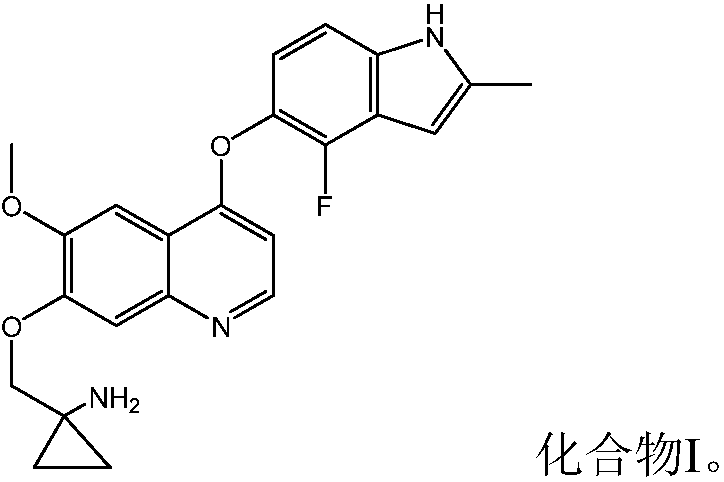
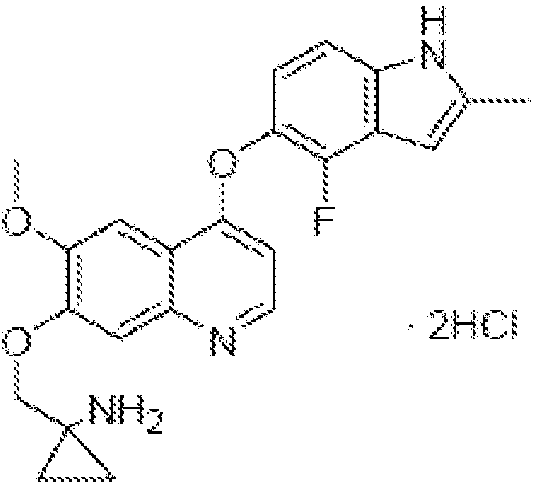
[0087] Example 1 1-[[[4-(4-Fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl]oxy]methyl] Cyclopropylamine dihydrochloride
[0088]
[0089] 1-[[[4-(4-Fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl was prepared with reference to the method of Example 24 in WO2008112407 ]oxy]methyl]cyclopropylamine, and then according to the preparation method of "Example in salt form" in the specification of WO2008112407, the title compound was prepared.
[0090] Or prepared by referring to the method disclosed in Chinese patent application CN102344438A.
Embodiment 21
[0091] Example 2 1-[[[4-(4-Fluoro-2-methyl-1H-indol-5-yl)oxy-6-methoxyquinolin-7-yl]oxy]methyl] Preparation of capsules of cyclopropylamine dihydrochloride (dihydrochloride of compound I)
[0092]
[0093]
[0094] The dihydrochloride of compound I is pulverized, crossed with 80 mesh sieves; then mixed with mannitol and hydroxypropyl cellulose; then added the microcrystalline cellulose of the recipe quantity, mixed homogeneously, crossed the 0.8mm sieve; finally added the recipe quantity of magnesium stearate, mix well and fill the capsules.
[0095] For capsules with other contents of the dihydrochloride of compound I, it can be prepared with reference to the same proportion and prescription above.
Embodiment 3
[0096] Example 3 Clinical trial
[0097] A clinical trial of Compound I dihydrochloride capsules was conducted in female patients ≥18 years of age with advanced or metastatic triple-negative breast cancer (ER-negative, PR-negative, HER2-negative) with measurable disease (according to RECIST 1.1), patients Received or not previously received chemotherapy, compound I dihydrochloride capsules alone or in combination with chemotherapy, administered at 12 mg, once a day, according to the regimen of continuous administration for two weeks and one week off, that is, every 3 One week is a medication cycle. Evaluation indicators include efficacy indicators: progression-free survival (PFS), objective response rate (ORR), duration of response (DOR), stable disease (SD) rate, clinical benefit rate (CBR), overall survival (OS), etc. ; Safety indicators: incidence and severity of adverse reactions; quality of life, etc.
[0098] Clinical trial results:
[0099] The dihydrochloride salt o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com