Larotrectinib hydrochloride, and preparation method and application thereof
A technology for larotrectinib hydrochloride and larotrectinib hydrochloride, which is applied in the field of medicinal chemistry, can solve the problems of inability to form a good solid powder crystal form, and achieves the effects of good stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 500g of (3S)-N-[5-[(2R)-2-(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-A]pyrimidine-3- Base]-3-hydroxyl-1-pyrrolidinecarboxamide was dissolved in 3000ml of ethanol, cooled to 5°C with stirring, and 120ml of concentrated hydrochloric acid (mass fraction was 36%) was added dropwise. 5h, suction filtration to obtain (3S)-N-[5-[(2R)-2-(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-A]pyrimidine- I-HC crystal form of 3-yl]-3-hydroxy-1-pyrrolidinecarboxamide hydrochloride 520g yellow solid powder.
Embodiment 2
[0047] 5g of (3S)-N-[5-[(2R)-2-(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-A]pyrimidine-3- Base]-3-hydroxy-1-pyrrolidinecarboxamide was dissolved in 20ml of ethanol, stirred and cooled down to -5°C, added 1.5ml of concentrated hydrochloric acid, kept stirring at 20°C for 4h, and obtained (3S)-N-[ 5-[(2R)-2-(2,5-Difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-A]pyrimidin-3-yl]-3-hydroxy-1-pyrrole I-HC crystal form of alkanecarboxamide hydrochloride 5.1g yellow solid powder.
Embodiment 3
[0049]50g of (3S)-N-[5-[(2R)-2-(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-A]pyrimidine-3- Base]-3-hydroxy-1-pyrrolidinecarboxamide was dissolved in 250ml of isopropanol, stirred and cooled down to 0°C, added 20ml of concentrated hydrochloric acid, kept stirring at 18°C for 2h, and obtained (3S)-N-[ 5-[(2R)-2-(2,5-Difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-A]pyrimidin-3-yl]-3-hydroxy-1-pyrrole I-HC crystal form of alkanecarboxamide hydrochloride 45g yellow solid powder.
[0050] (1) Characterization:
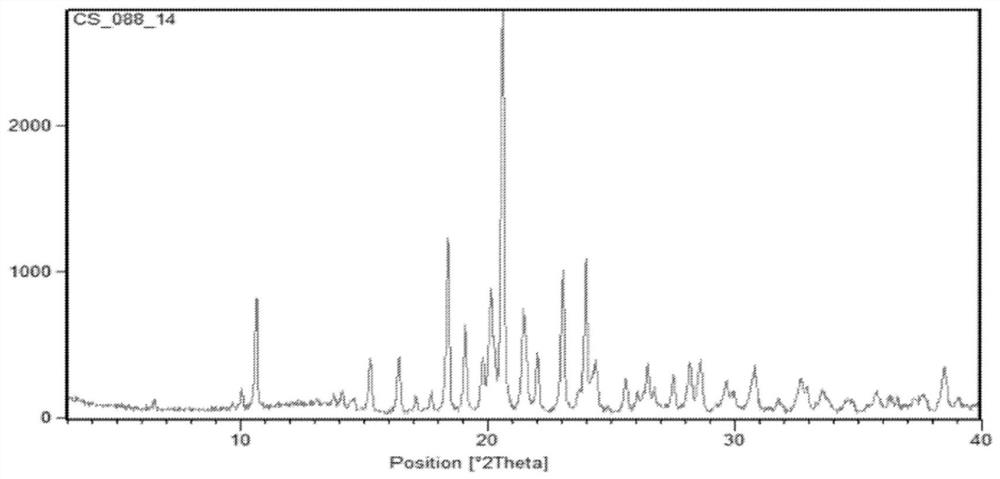
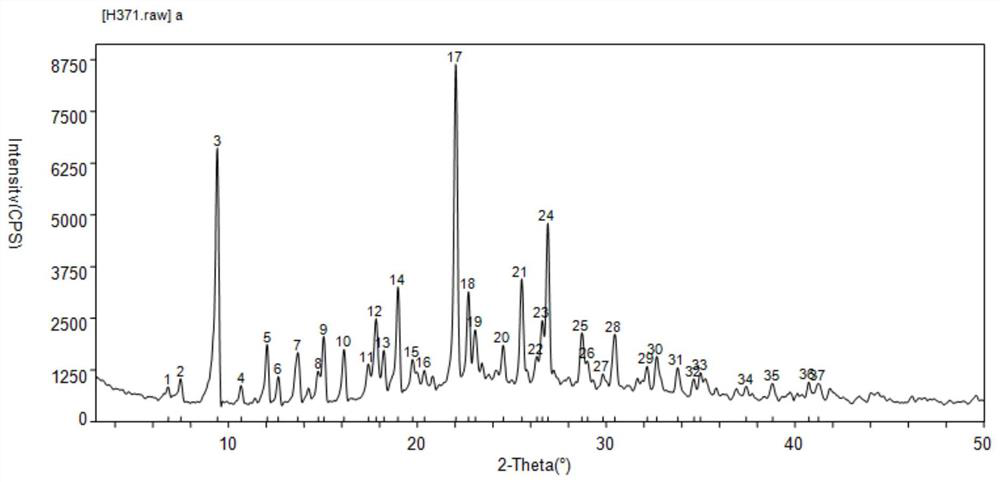
[0051] figure 2 (3S)-N-[5-[(2R)-2-(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-A]pyrimidin-3-yl] -XRPD pattern of 3-hydroxy-1-pyrrolidinecarboxamide hydrochloride (larotrenib hydrochloride) (I-HC crystal form). PeakSearch Report(37Peaks, Max P / N=16.4)[H371.raw]a PEAK: 19-pts / Parabolic Filter, Threshold=9.0, Cutoff=2.0%, BG=7 / 1.0, Peak-Top=Summit.
[0052] Table 1 is the X-ray diffraction chart of the crystal form of larotrectinib hydrochloride. image 3 (3S)-N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com