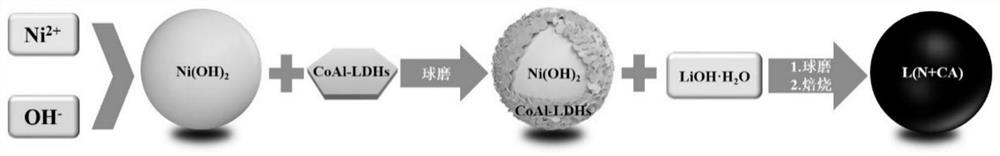
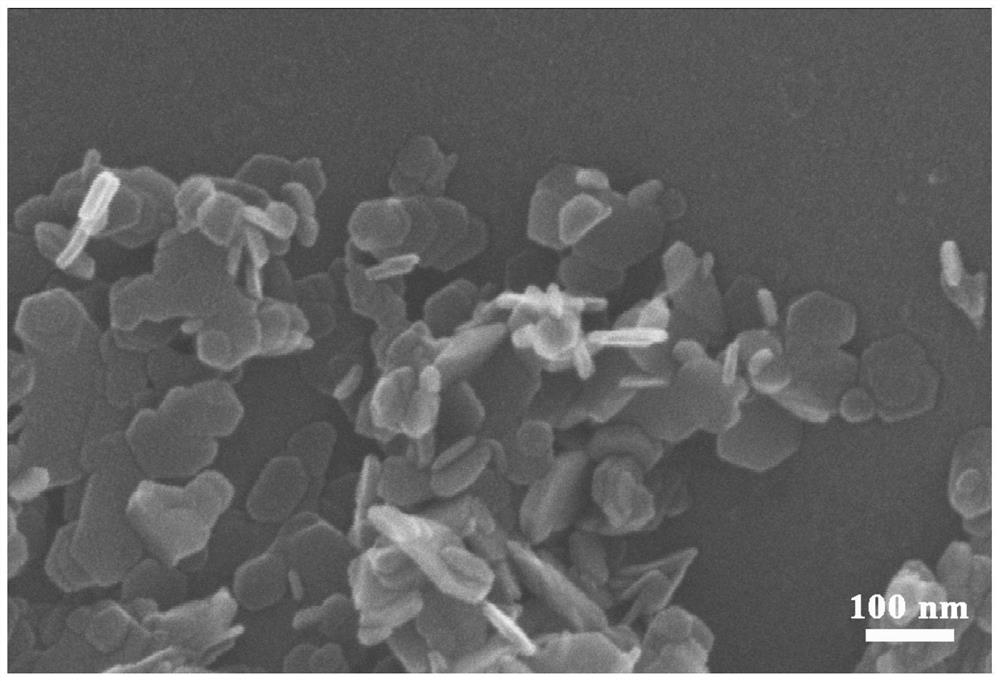
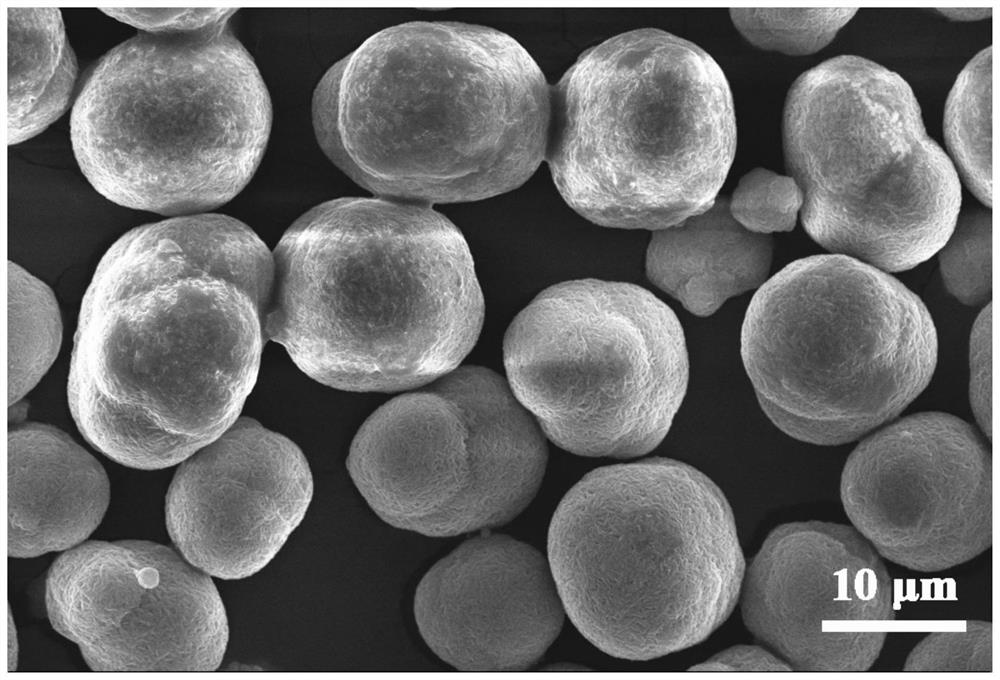
Method for preparing lithium nickel cobalt aluminate positive electrode material
A technology of nickel cobalt lithium aluminate and cathode material, which is applied in nanotechnology, chemical instruments and methods, and nickel compounds for materials and surface science, and can solve the problem of increased raw material cost, high control accuracy requirements, and difficult synthesis techniques. big problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) Preparation of spherical nickel hydroxide: prepare a nickel sulfate solution with a nickel ion concentration of 2mol / L, an ammonia solution with an ammonia concentration of 85g / L, and an alkali solution with a sodium hydroxide concentration of 6mol / L; Dilute the 85g / L ammonia solution to 7g / L and fill the 10L overflow reactor as the bottom liquid of the reaction. Maintain the stirring speed of the overflow reactor at 600rpm and the temperature of the reactor at 55℃; use a constant flow rate of 500mL / h to A nickel sulfate solution with a concentration of 2mol / L is added to the overflow reactor, and an ammonia solution with an ammonia concentration of 85g / L is added to the overflow reactor to maintain the ammonia concentration in the reaction solution at 7g / L, while adding a concentration of 6mol / L of sodium hydroxide solution and adjust the pH value of the reaction solution by controlling the flow rate. At the beginning of the reaction, maintain the pH value of the re...
Embodiment 2
[0063] (1) Preparation of spherical nickel hydroxide: prepare a nickel nitrate solution with a nickel ion concentration of 1.5 mol / L, prepare an ammonia solution with an ammonia concentration of 50 g / L, and prepare an alkali solution with a sodium hydroxide concentration of 5 mol / L; Ammonia solution with a concentration of 50g / L is diluted to 6g / L and filled with a 10L overflow reactor as the bottom liquid of the reaction. The stirring speed of the overflow reactor is maintained at 500rpm and the temperature of the reactor is 50℃; at a constant flow rate of 450mL / h Add a nickel nitrate solution with a concentration of 1.5mol / L to the overflow reactor, and at the same time add an ammonia solution with an ammonia concentration of 50g / L to the overflow reactor to maintain the ammonia concentration in the reaction solution at 6g / L, while adding the concentration It is a 5mol / L sodium hydroxide solution and the pH value of the reaction solution is adjusted by controlling the flow rat...
Embodiment 3
[0068] (1) Preparation of spherical nickel hydroxide: prepare a nickel acetate solution with a nickel ion concentration of 2.5 mol / L, an ammonia solution with an ammonia concentration of 70 g / L, and an alkali solution with a sodium hydroxide concentration of 7 mol / L; Ammonia solution with a concentration of 70g / L is diluted to 8g / L and filled with a 10L overflow reactor as the bottom liquid of the reaction. The stirring speed of the overflow reactor is maintained at 400rpm and the temperature of the reactor is 60℃; at a constant flow rate of 400mL / h Add a nickel acetate solution with a concentration of 2.5mol / L to the overflow reactor, and at the same time add an ammonia solution with an ammonia concentration of 70g / L to the overflow reactor to maintain the ammonia concentration in the reaction solution at 8g / L, while adding the concentration It is a 7mol / L sodium hydroxide solution and the pH value of the reaction solution is adjusted by controlling the flow rate. At the beginn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Radial size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com