Production method of 8-hydroxyquinoline
A technology of hydroxyquinoline and production method, applied in the direction of organic chemistry, etc., can solve the problems of 8-hydroxyquinoline product yield and quality impact, poor product quality, low product yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
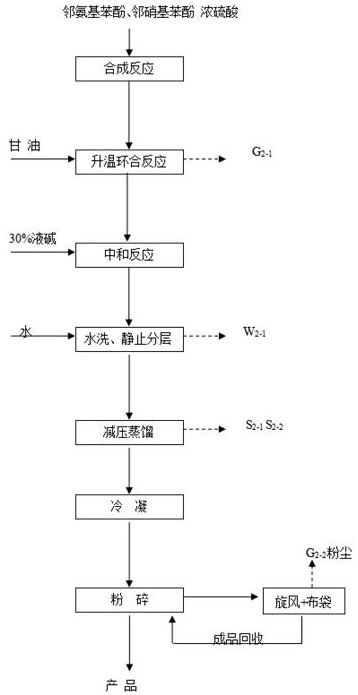
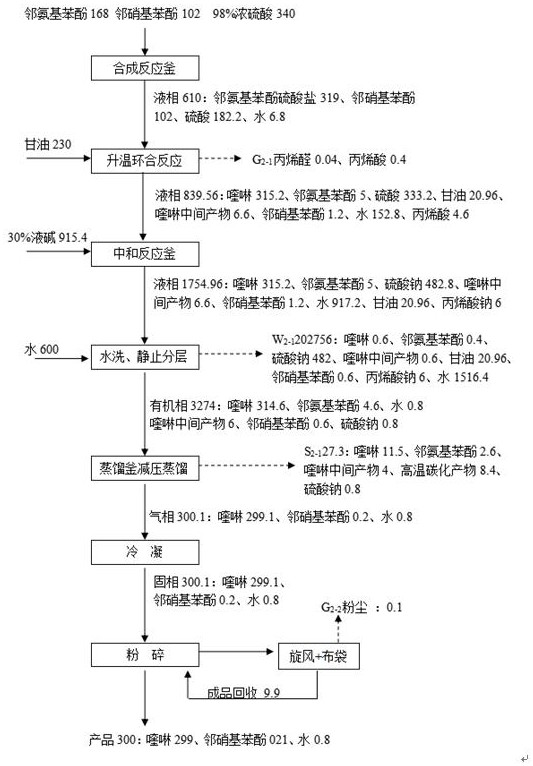
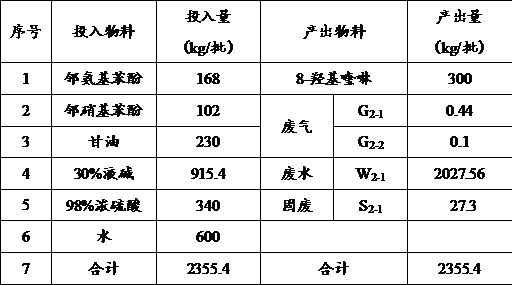
[0033] as attached figure 1 Shown, this embodiment provides a kind of production method of 8-hydroxyquinoline, it may further comprise the steps:
[0034] S1. After putting 168kg of o-aminophenol and 102kg of o-nitrophenol into the mixing kettle, add 340kg of 98% sulfuric acid dropwise into the mixing kettle. After the dropwise addition, stir and mix for half an hour. During the stirring process, the temperature should be controlled at 70 °C, after the stirring and mixing are completed, a mixture is obtained.
[0035] S2. Vacuum transfer the above mixture to the synthesis kettle, then, drop 230kg of glycerin in the metering tank into the synthesis kettle (dropping is completed within 8 hours), and heat up to 150°C through the jacket of the synthesis kettle to carry out The cyclization reaction was carried out for 4 hours. After the reaction was completed, circulating cooling water was passed through the jacket of the synthesis kettle to lower the temperature of the material t...
Embodiment 2
[0039] This embodiment provides a kind of production method of 8-hydroxyquinoline, it may further comprise the steps:
[0040] S1. After putting 168kg of o-aminophenol and 84kg of o-nitrophenol into the mixing kettle, add 302.4kg of 98% sulfuric acid dropwise into the mixing kettle. After the addition, stir and mix for half an hour. 60°C, after stirring and mixing, a mixture was obtained.
[0041] S2. Vacuum transfer the above mixture to the synthesis kettle, then add 201.6kg of glycerin in the metering tank dropwise into the synthesis kettle (the addition is completed within 8 hours), and heat up to 140°C through the jacket of the synthesis kettle The cyclization reaction was carried out for 4 hours. After the reaction was completed, circulating cooling water was passed through the jacket of the synthesis kettle to lower the temperature of the material to 80° C. to obtain a reaction solution.
[0042] S3. Pump the above reaction solution into the neutralization kettle, then ...
Embodiment 3
[0045] This embodiment provides a kind of production method of 8-hydroxyquinoline, it may further comprise the steps:
[0046] S1. After putting 168kg of o-aminophenol and 117.6kg of o-nitrophenol into the mixing kettle, add 369.6kg of 95% sulfuric acid dropwise into the mixing kettle. After the dropwise addition, stir and mix for half an hour. The temperature needs to be controlled during the stirring process After stirring and mixing at 80° C., a mixture was obtained.
[0047] S2. Vacuum transfer the above mixture to the synthesis kettle, then add 252kg of glycerin in the metering tank dropwise into the synthesis kettle (the dropwise addition is completed within 8 hours), and heat up to 160°C through the jacket of the synthesis kettle to carry out The cyclization reaction was carried out for 4 hours. After the reaction was completed, circulating cooling water was passed through the jacket of the synthesis kettle to lower the temperature of the material to 80° C. to obtain a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com