Crystallization and purification method of favipiravir key intermediate 3, 6-difluoropyrazine-2-carbonitrile
A difluoropyrazine and purification method technology, applied in the direction of organic chemistry, etc., can solve the problems of difficult recovery, large amount of organic solvent, cumbersome operation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
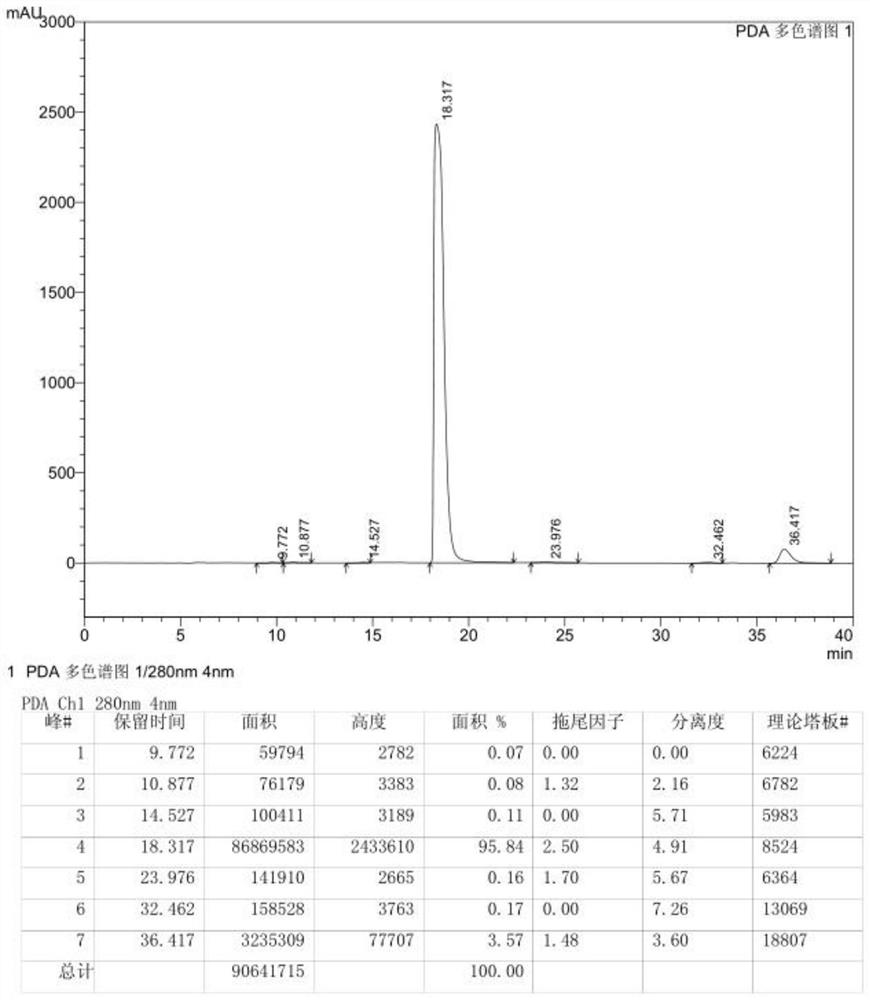
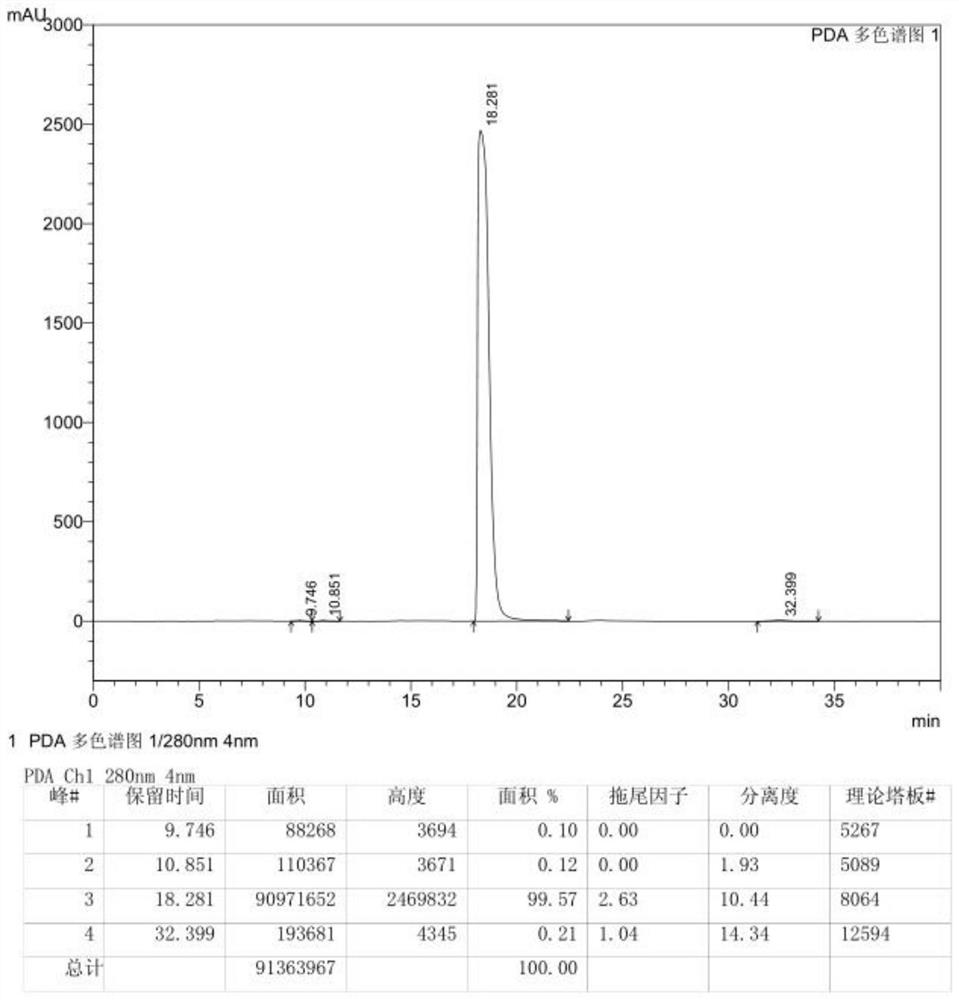
[0030] Take 20.0g of crude 3,6-difluoropyrazine-2-carbonitrile and add it to 60.0g of ethyl tert-butyl ether, stir, add 10.0g of column chromatography silica gel, heat to 50°C, stir for 0.5h to decolorize, pump After filtration, the obtained clear filtrate was cooled to -20~-15°C within 0.5~1h, stirred at -20~-15°C for 0.5h and then filtered, and the obtained solid was washed twice with ethyl tert-butyl ether, each time 5.0g. The obtained solid was vacuum-dried to dryness at 20° C. to obtain 17.9 g of 3,6-difluoropyrazine-2-carbonitrile. 99.52% purity.
Embodiment 2
[0032] Add 20.0g of crude 3,6-difluoropyrazine-2-carbonitrile to 300.0g of ethyl tert-butyl ether, stir, add 100.0g of silica gel for column chromatography, heat to 65°C, stir for 3h to decolorize, and filter with suction , the obtained clear filtrate was cooled to 15-20°C within 4-5h, stirred at 15-20°C for 6h and then filtered, and the obtained solid was washed twice with ethyl tert-butyl ether, 50g each time. The obtained solid was vacuum-dried to dryness at 30° C. to obtain 13.5 g of 3,6-difluoropyrazine-2-carbonitrile. 99.56% pure.
Embodiment 3
[0034] Take 20.0g of crude 3,6-difluoropyrazine-2-carbonitrile and add it to 80.0g of ethyl tert-butyl ether, stir, add 20.0g of silica gel for column chromatography, heat to 55°C, stir for 0.5h to decolorize, pump Filter, cool the obtained clear filtrate to -10~-5°C within 1~2h, stir at -10~-5°C for 1h and then filter, the obtained solid is washed twice with ethyl tert-butyl ether, each time 10.0 g. The obtained solid was vacuum-dried to dryness at 25°C to obtain 17.3 g of 3,6-difluoropyrazine-2-carbonitrile. 99.55% pure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com