Intranasal delivery of levodopa power by precision olfactory device
A levodopa, powder technology, applied in the field of intranasal delivery of levodopa powder through a precision nasal device, can solve the problems of difficult patient management, high incidence, tolerance problems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
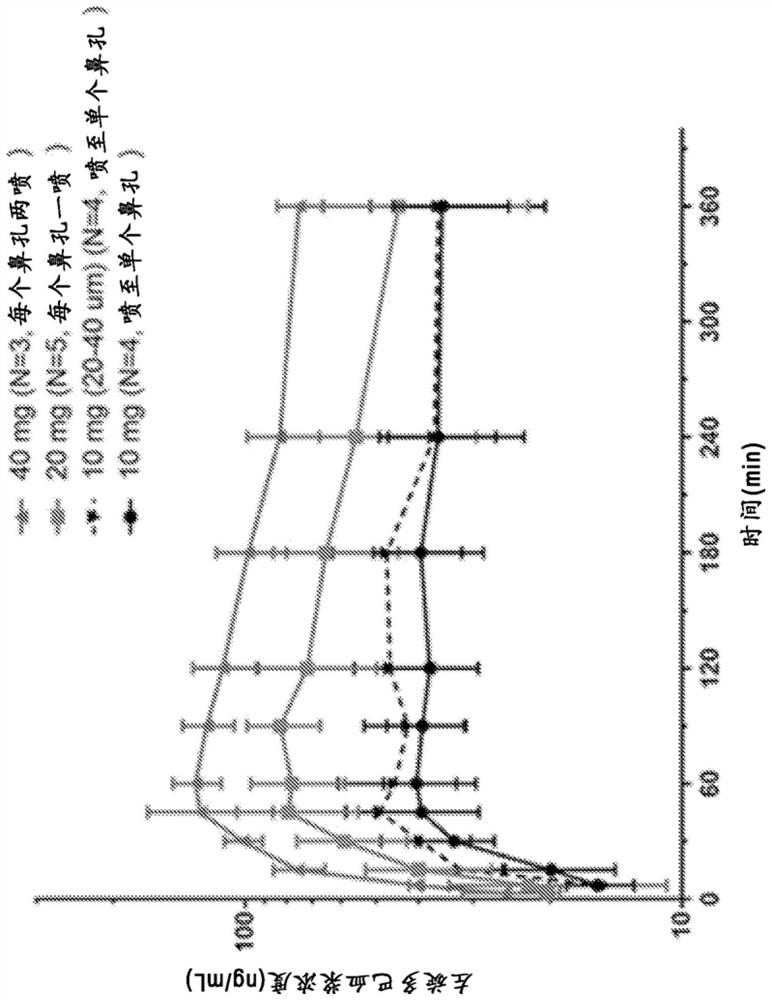
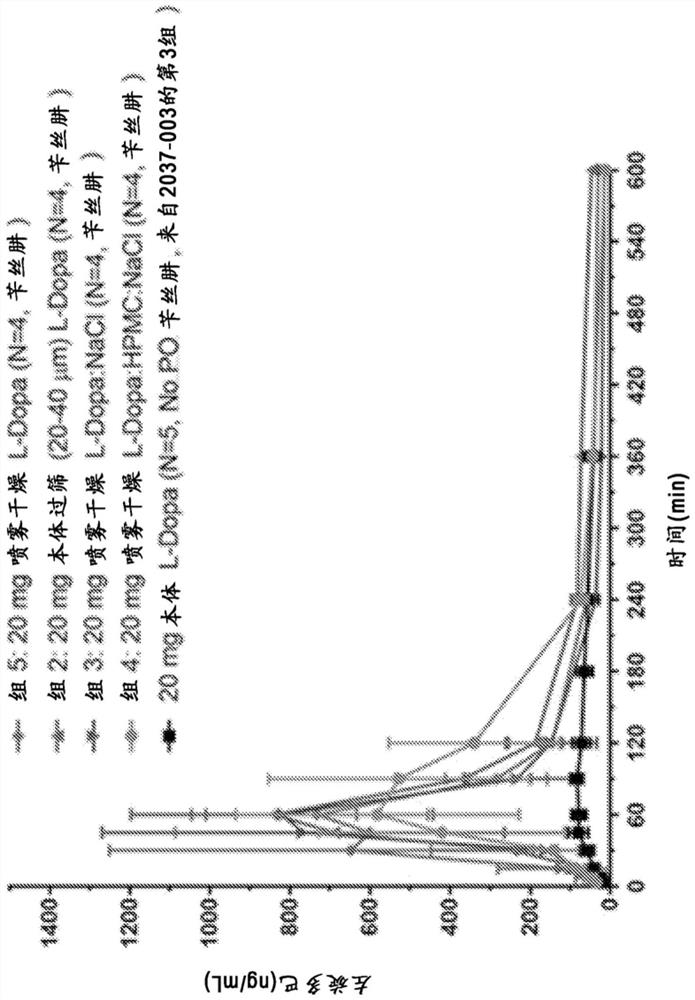
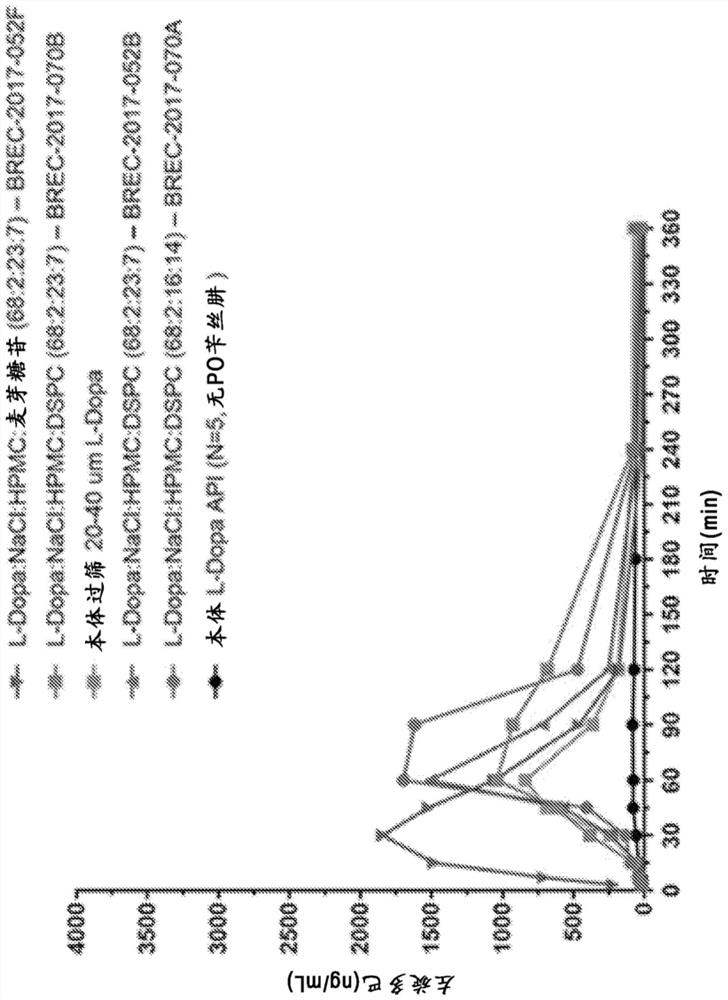
[0143] Example 1: Non-human primate PK studies
[0144] A series of L-DOPA (levodopa) powder formulations were developed and manufactured to evaluate the pharmacokinetics of intranasal administration of levodopa in non-human primates ("NHP"). The goal of powder formulation development was to achieve a formulation that would result in a rapid increase in plasma concentration to >200 ng / mL, preferably >400 ng / mL after intranasal delivery using a non-human primate precision nasal delivery ("nhpPOD") device, So that the preparation is expected to positively affect the "OFF" event in Parkinson's disease.
[0145] Four single-dose PK studies were performed in cynomolgus monkeys to examine PK following administration of a multipowder L-DOPA formulation delivered by the intranasal route using the nhpPOD device. Formulations examined included unmodified crystalline powder (median particle size ~50 μm), sieved formulations containing crystalline L-DOPA particles in a defined size range...
Embodiment 2
[0244] Example 2: Phase IIa, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Safety and Pharmacokinetics of INP103 (PODL-DOPA) Administered to Levodopa-Responsive Parkinson's Disease Patients in the Presence of Benserazide Kinetic / pharmacodynamic studies
[0245] Research design
[0246] A powder formulation of L-DOPA (levodopa) was tested in a randomized, double-blind, placebo-controlled, single ascending dose study to demonstrate precision nasal delivery via I231 Safety, tolerability and PK / pharmacodynamics of L-DOPA delivered by device to human subjects. The I231 POD device is a hand-held, manually actuated, propellant-driven, metered-dose administration device designed to deliver powder pharmaceutical formulations to the nasal cavity.
[0247] Intranasal administration was performed as single ascending doses of L-DOPA administered (aspirated) once (35 mg), twice (70 mg) or four times (140 mg) into the nostrils. L-DOPA was administered 60 minutes af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Median diameter | aaaaa | aaaaa |
| Median diameter | aaaaa | aaaaa |
| Median diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com