Application of otilonium bromide to preparation of antitumor medicines
An anti-tumor drug, octilonium bromide technology, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations and other directions, can solve the problems of no patent application, no report on the effect of tumor proliferation and growth, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
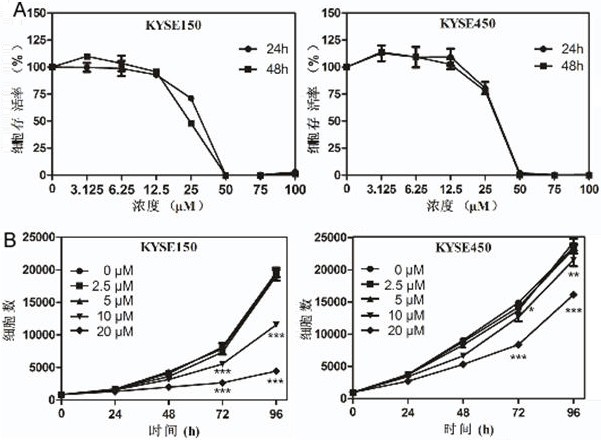
[0040] Example 1 In vitro experiment of otilonium bromide inhibiting the proliferation of esophageal cancer cells
[0041] Cytotoxicity test
[0042] The experimental process is: KYSE150 cells were treated with 8×10 3 cells / well were inoculated in a 96-well plate for culture (10% FBS / 1640, 37°C, 5% CO 2 ), KYSE450 cells at 1.2×10 4 Each well was inoculated in a 96-well plate for culture (10% FBS / DMEM, 37°C, 5% CO 2 ), after 14-16 hours, replace the fresh medium and add otilonium bromide (DMSO solution), so that the final concentration of otilonium bromide in the medium is 0 μM, 3.125 μM, 6.25 μM, 12.5 μM, 25 μM, 50 μM, 75 μM, 100 μM, cultured for 24 and 48 hours respectively, the cells were taken out of the incubator, the original medium was discarded, and washed twice with 1×PBS. Then discard the PBS, add 100 μL / well of 4% paraformaldehyde, fix for 30 minutes, discard the paraformaldehyde, and wash twice with 1×PBS. Then discard the PBS, stain with DAPI (DAPI stock solut...
Embodiment 2
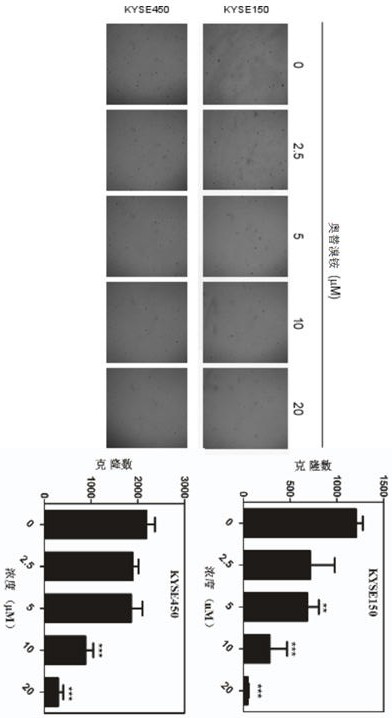
[0047] Example 2 In vitro experiments of otilonium bromide inhibiting the ability of esophageal cancer cell colony formation
[0048] The experimental process is as follows: spread 3 mL of BME medium (containing 10% FBS and 0.5% agar) in each well of a 6-well plate, and spread suspension of esophageal cancer cells (KYSE150: 8×10 3 pcs / hole or KYSE450: 8×10 3 pcs / well) top layer gel (the top layer gel contains 1 mL BME medium, the medium contains 10% FBS and 0.33% agar, and the final concentrations of drugs in the medium are 0 μM, 2.5 μM, 5 μM, 10 μM, 20 μM), in an incubator (37°C, 5% CO 2 ) After 7-14 days of culture or according to the condition of the cells, when clones are formed, use IN Cell Analyzer 3000 to count the clones ( figure 2 For the results obtained after culturing for 10 days).
[0049] Experimental results such as figure 2 shown. from figure 2 It can be seen that the number of cell clones decreased after treatment with otilonium bromide, and the size ...
Embodiment 3
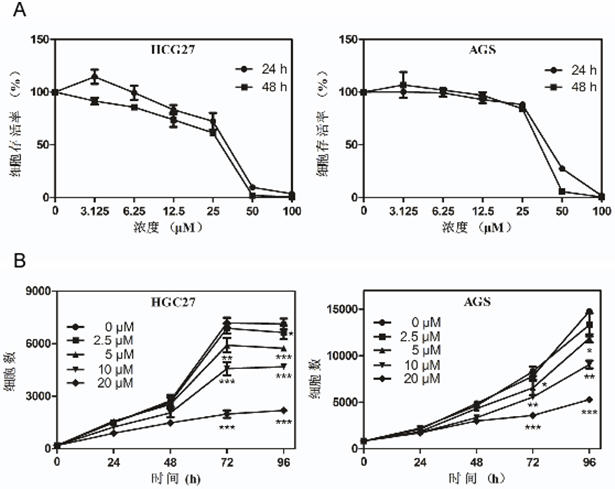
[0050] Example 3 In Vitro Experiment of Otilonium Bromide Inhibiting the Proliferation of Gastric Cancer Cells
[0051] Cytotoxicity test
[0052] The experimental process is: HGC27 cells were divided into 6×10 3 cells / well were inoculated in a 96-well plate for culture (10% FBS / 1640, 37°C, 5% CO 2 ), AGS cells in 8×10 3 Each well was inoculated in a 96-well plate for culture (10% FBS / F12k, 37°C, 5% CO 2 ), after 14-16 hours, replace the fresh medium and add otilonium bromide (DMSO solution), so that the final concentration of otilonium bromide in the medium is 0 μM, 3.125 μM, 6.25 μM, 12.5 μM, 25 μM, 50 μM, 100 μM), cultured for 24 and 48 hours respectively, the cells were taken out of the incubator, the original medium was discarded, and washed twice with 1×PBS. Then discard the PBS, add 100 μL / well of 4% paraformaldehyde, fix for 30 minutes, discard the paraformaldehyde, and wash twice with 1×PBS. Then discard the PBS, stain with DAPI (DAPI stock solution: 1×PBS=1:5000...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com