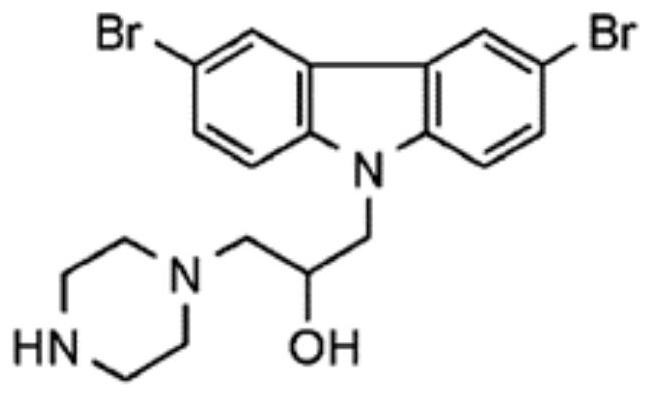
Application of iMAC1 in preparation of product for treating cancer
A cancer, product technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
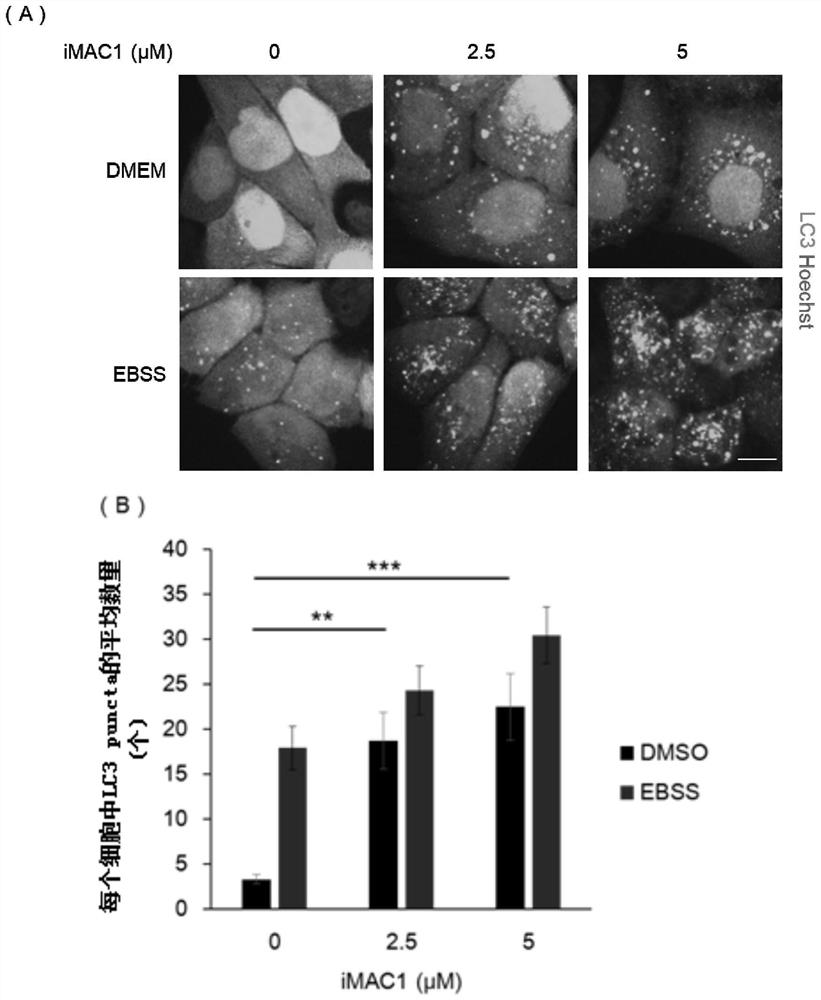
[0039] Example 1, iMAC1 increases intracellular LC3 punctate aggregation
[0040] LC3 protein is a classic marker protein for evaluating autophagy and an important marker on the bilayer membrane of autophagosomes. If the punctate aggregation (LC3 puncta) of intracellular LC3 protein increases, the autophagy level of the cell increases.
[0041] 1. Take a 24-well plate, put a round cover glass in each well, and then inoculate GFP-LC3-HeLa cells for adherent culture.
[0042] 2. After completing step 1, add iMAC1 to each well to obtain system 1; in system 1, the concentration of iMAC1 is 0 μM, 2.5 μM or 5 μM.
[0043] 3. After completing step 2, take system 1 and place it at 37°C, 5% CO 2 Incubate for 2 h in a cell culture incubator.
[0044] 4. Take system 1 after step 3, discard the liquid phase, and add iMAC1-containing EBSS (Earle's Balanced Salt Solution) solution (as a positive control) or iMAC1-containing DMEM medium to obtain system 2. In the EBSS solution containing i...
Embodiment 2
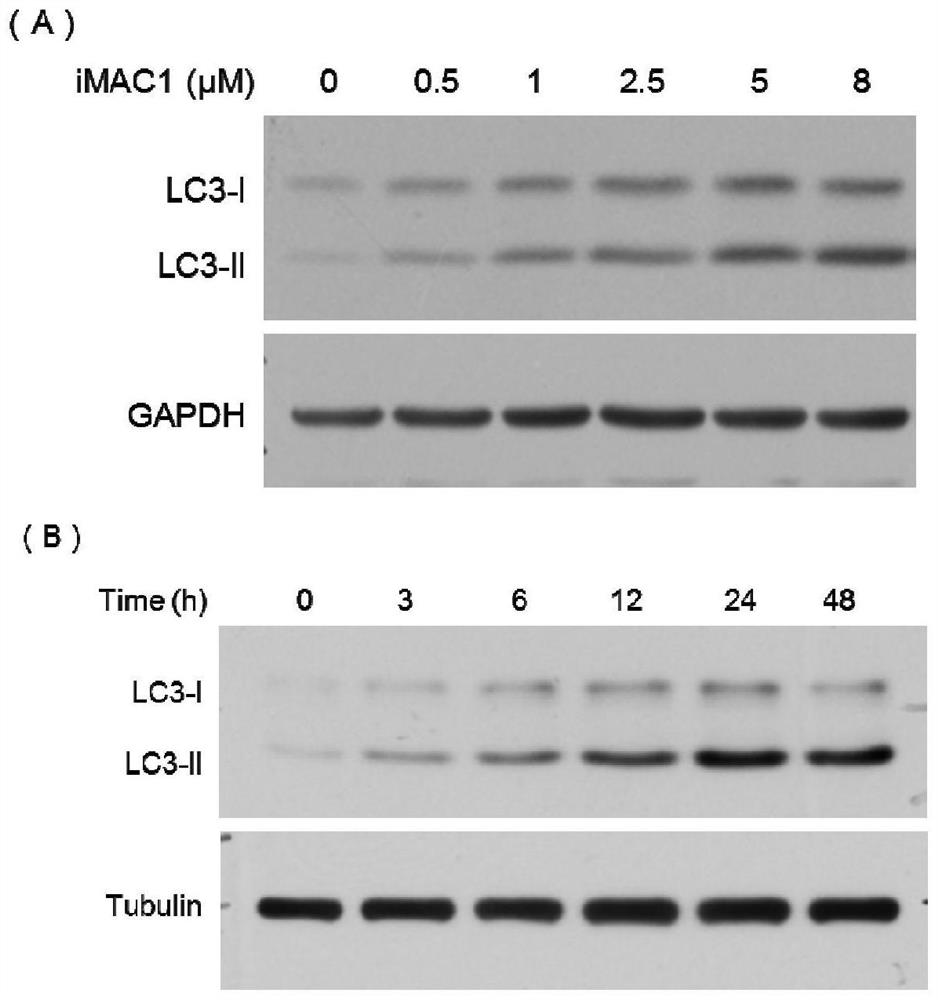
[0055] Example 2, iMAC1 promotes the accumulation of autophagosomes in a dose- and time-dependent manner
[0056] LC3-II is the esterified form of LC3-I, which can anchor on the bilayer membrane of autophagosomes and serve as an important marker of autophagosomes.
[0057] 1. In order to study the relationship between the level of autophagy and the dose of iMAC1, the inventors of the present invention used Western blotting to detect the changes in the abundance of LC3-II protein. Specific steps are as follows:
[0058] (1) Add iMAC1 to adherent cultured HeLa cells to obtain system 1; in system 1, the concentration of iMAC1 is 0 μM, 0.5 μM, 1.0 μM, 2.5 μM, 5.0 μM or 8.0 μM.
[0059] (2) After completing step (1), take system 1 and place it at 37°C, 5% CO 2 cultured in a cell culture incubator for 24 h.
[0060] (3) After completing step (2), extract the total protein of each cell, and then use the antibody of LC3 protein or GAPDH antibody (control) as the primary antibody fo...
Embodiment 3
[0068] Example 3, iMAC1 inhibits the downstream of the autophagic flow of cells
[0069] 1. Western blotting
[0070] (1) Prepare system 1-system 4.
[0071] Take adherent cultured HeLa cells and add Baf-A1 and iMAC1 to obtain system 1. In system 1, the concentration of Baf-A1 was 100 nM, and the concentration of iMAC1 was 5.0 μM.
[0072] Take adherent cultured HeLa cells and add Baf-A1 and DMSO to obtain system 2. In system 2, the concentration of Baf-A1 was 100 nM.
[0073] Take adherent cultured HeLa cells and add DMSO and iMAC1 to obtain system 3. In system 3, the concentration of iMAC1 was 5.0 μM.
[0074] Take adherent cultured HeLa cells and add DMSO to obtain system 4.
[0075] (2) After completing step (1), take system 1-system 4 respectively and place them at 37°C and 5% CO 2 cultured in a cell culture incubator for 24 h.
[0076] (3) After completing step (2), extract the total protein of each cell, and then use the antibody of LC3 protein or Tubulin antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com