BP180 IgE antibody concentration detection kit and detection method and application thereof
A technology for detecting kits and antibody concentrations, applied in the field of BP180IgE antibody concentration detection kits, which can solve the problems of disease severity assessment and no NBP-specific detection methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Secondly, this protocol provides a reagent preparation method for detecting BP180 IgE antibody detection ELISA kit in serum, including:
[0046] 1) Prokaryotic expression of BP180 NC16A antigen
[0047] (1) Preparation of Competent Cells
[0048] ① After LB solid culture and autoclaving, cool to about 60°C, add ampicillin to make the final concentration reach 100 μg / mL, shake well and plate.
[0049] ②Pick a single colony from the reactivated E.coli DH5α plate, inoculate it in 3-5mL LB liquid medium, culture at 37°C with shaking at 220rmp until logarithmic growth (A600 0.6-0.8 measured by spectrophotometer, about 12h).
[0050] ③Transfer the bacterial suspension into 100mL LB liquid medium at a ratio of 1:100 to 1:50, and incubate with shaking at 37°C and 220rmp for about 3 hours until A600=0.5.
[0051] ④Transfer the culture solution into a centrifuge tube (8mL / tube), place on ice for 10-20 minutes, and then centrifuge at 3000g for 10min at 4°C.
[0052] ⑤ Discard t...
Embodiment
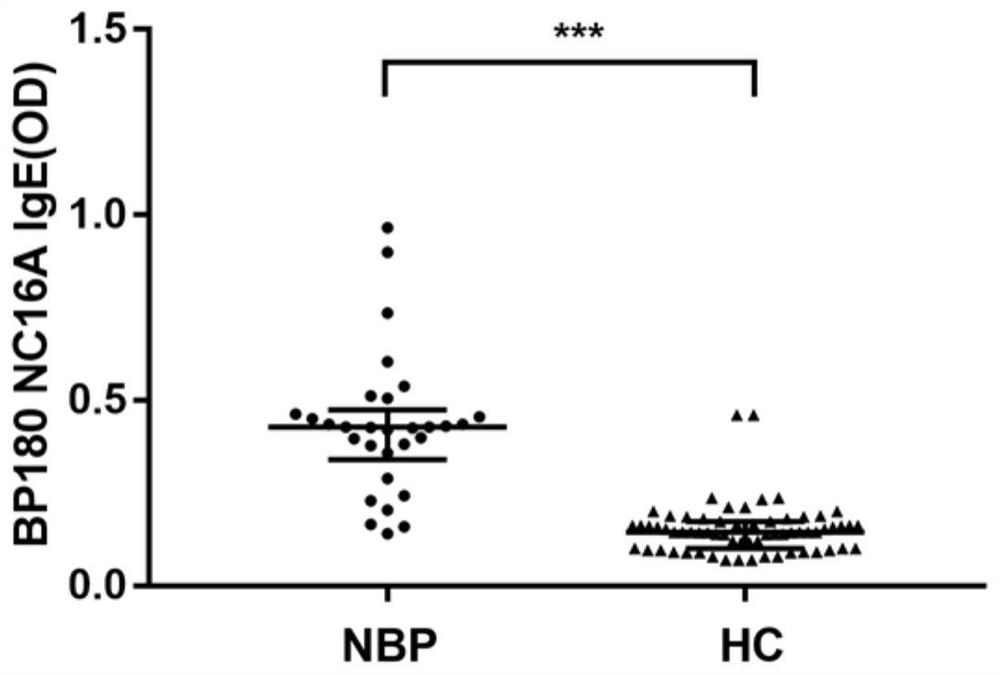
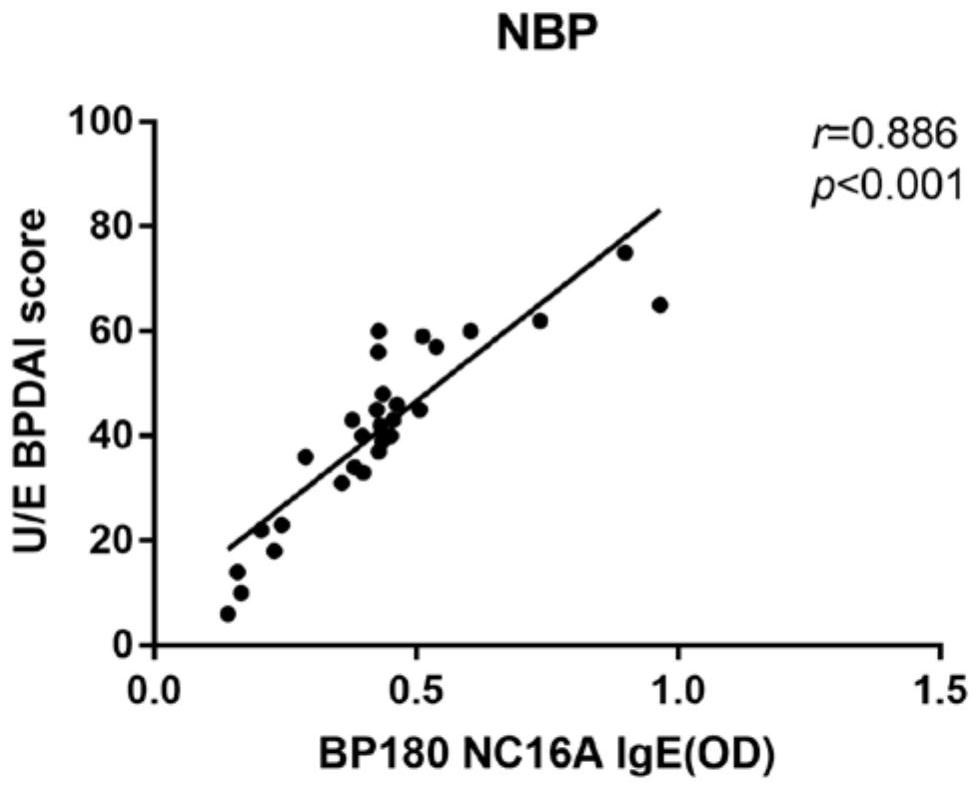
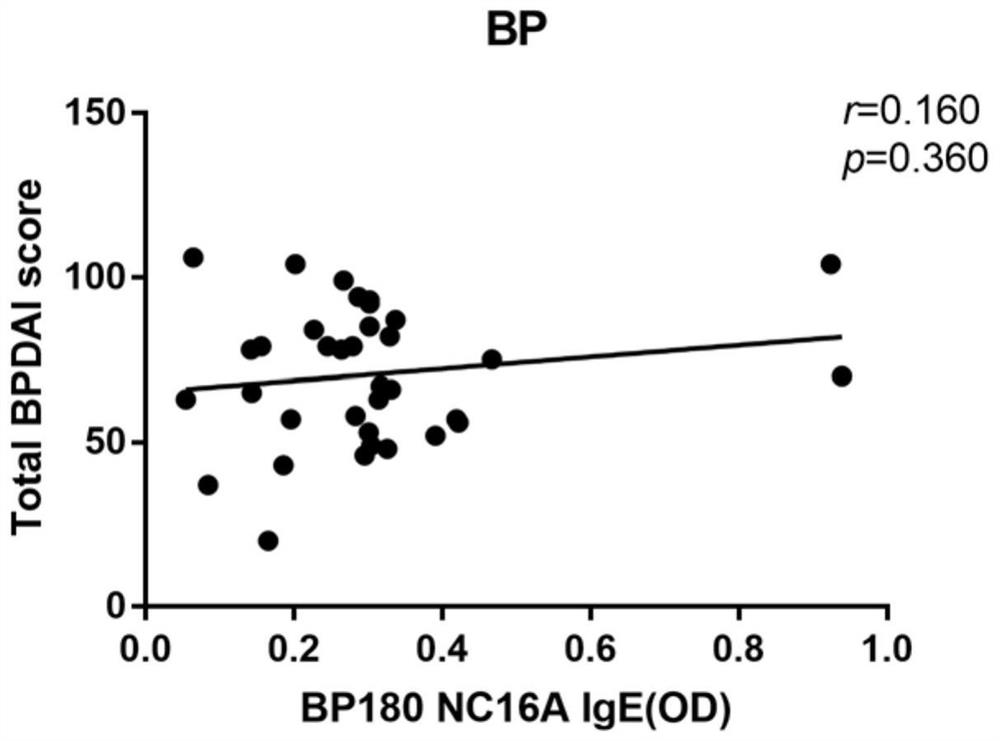
[0134] Example: Study on the correlation between BP180 IgE antibody level and NBP condition
[0135] 1. Research object
[0136] The 35 cases of typical BP patients and 30 cases of NBP patients included in this study were first-time diagnosed patients in the Department of Dermatology of the First Affiliated Hospital of Dalian Medical University. They were all in the active stage of the disease and before receiving any systemic treatment. All patients were diagnosed by experienced dermatologists based on clinical manifestations, histopathological examinations, and direct and indirect immunofluorescence examinations and met the BP diagnostic criteria established by the British Skin Association in 2012. For each patient, after being informed of the purpose of the study, he agreed to donate his blood for this study and signed an informed consent form. Patients with pregnancy, other autoimmune diseases, other skin diseases, asthma, parasitic infections, hypereosinophilic syndrome,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com