Preparation method of citronellyl cyanide
A technology of citronellyl nitrile and citronellic acid, which is applied in the field of citronellyl nitrile preparation to achieve the effects of ensuring intrinsic safety, mild chemical properties and low risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
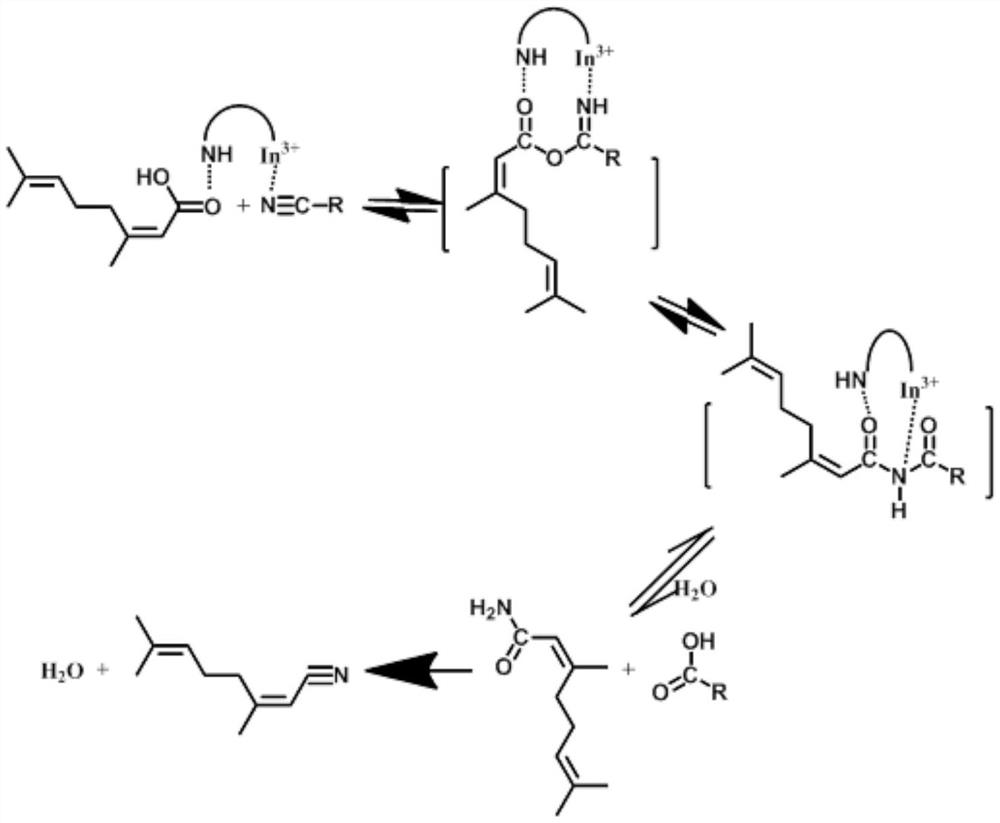
[0068] 1) Add 71.16ml polyvinyl alcohol aqueous solution (concentration 0.08g / ml, polyethylene 0.129mol) into 5ml indium trichloride aqueous solution (concentration 0.6g / ml, indium element 0.016mol) under vigorous stirring, Stir at -2 °C for 1 h; keep stirring, add 38.193 g of freshly prepared NaBH to the above solution 4 Aqueous solution (concentration 8wt%, wherein NaBH 4 0.08mol), forming a PVA-In nano-sol;
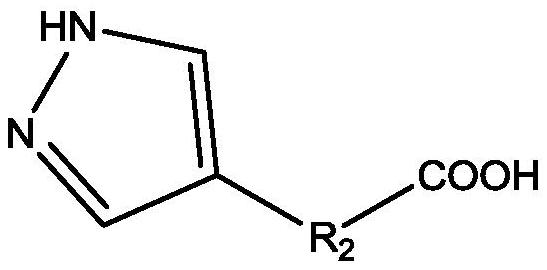
[0069] 2) Disperse 4.126g / 0.018mol 5-(1H-pyrazol-4-yl)isophthalic acid in 43.429ml water and vibrate ultrasonically (frequency 1500Hz) for 30h, then add to the PVA-In nano-sol prepared in step 1) , continue to stir at -2°C for 5h, then centrifuge, wash with deionized water three times, dry at 95°C for 3h, and then transfer to a roaster for 3.5h at 400°C to obtain the In-MOF catalyst. (The mass fraction of indium in the catalyst is about 18.2wt%)
Embodiment 2
[0071] 1) Add 64ml polyvinyl alcohol aqueous solution (concentration 0.1g / ml, polyethylene 0.145mol) into 3.75ml indium trichloride aqueous solution (concentration 0.8g / ml, indium element 0.016mol) under vigorous stirring, Stir at -1 °C for 1.5 h; keep stirring, add 37.344 g of freshly prepared NaBH to the above solution 4 Aqueous solution (concentration 9wt%, wherein NaBH 4 0.09mol), forming a PVA-In nano-sol;
[0072] 2) Disperse 3.952g / 0.021mol 3-(1H-pyrazol-4-yl)benzoic acid in 39.517ml of water and vibrate ultrasonically (frequency 1750Hz) for 33h, and then add it to the PVA-In nano-sol prepared in step 1), Continue to stir at -1°C for 5.5h, then centrifuge, wash with deionized water 4 times, dry at 100°C for 4h, and transfer to a roaster for 4h at 410°C to obtain the In-MOF catalyst. (The mass fraction of indium in the catalyst is about 17.985wt%)
Embodiment 3
[0074] 1) Add 33.55ml polyvinyl alcohol aqueous solution (concentration 0.1g / ml, polyethylene 0.076mol) to 3.75ml indium tribromide aqueous solution (concentration 0.8g / ml, indium element 0.008mol) under vigorous stirring , stirred at -1°C for 2 h; keeping stirring, added 19.56 g of freshly prepared NaBH to the above solution 4 Aqueous solution (concentration 9wt%, wherein NaBH 4 0.046mol), forming a PVA-In nano-sol;
[0075] 2) Disperse 2.554g / 0.014mol 5-(1H-pyrazol-4-yl)isophthalic acid in 25.54ml of water and vibrate ultrasonically (frequency 2000Hz) for 33h, then add to the PVA-In nano-sol prepared in step 1) , continue to stir at 0°C for 6h, then centrifuge, wash with deionized water five times, dry at 100°C for 4h, and then transfer to a roaster for 4h at 420°C to obtain In-MOF catalyst. (The mass fraction of indium in the catalyst is about 13.28wt%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com