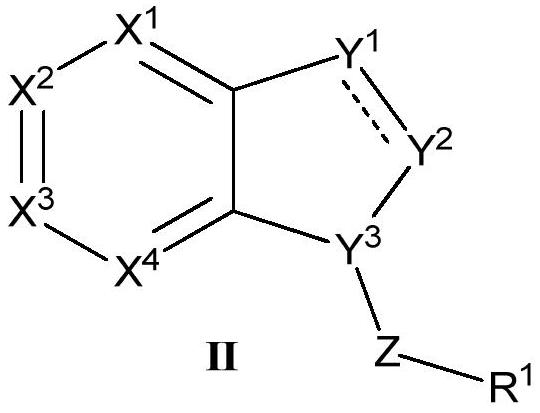
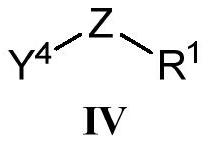
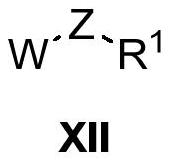Heterocyclic ring-containing compound, application thereof and composition containing heterocyclic ring-containing compound
A compound and pharmaceutical technology, which can be applied in the direction of active ingredients of heterocyclic compounds, drug combinations, and medical preparations containing active ingredients, and can solve problems such as single structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1211] Example 1.3-(1-propionylpiperidin-4-yl)benzo[d]oxazol-2(3H)-one (P1)
[1212]
[1213] Step 1.1. Synthesis of tert-butyl 4-(2-oxobenzo[d]oxazol-3(2H)-yl)piperidine-1-carboxylate
[1214]
[1215] CH 2 Cl 2 (300 mL) the solution was stirred and NaBH(OAc) was added 3 (32.4g, 151mmol), and stirred at room temperature for 14h, added CH 2 Cl 2 (1000mL) and saturated NaHCO 3 (1500mL) was diluted and the layers were separated. use CH 2 Cl 2 (2*500ml) extract the aqueous layer, wash the combined organic layer with brine, Na 2 SO 4 Dry and remove solvent under reduced pressure. CH 2 Cl 2 (300mL) was diluted, carbonyldiimidazole (18g, 110mmol) was added, stirred for 16h, washed with CH 2 Cl 2 (1L) and 1N HCl (1L), and the layers were separated. use CH 2 Cl 2 (2*500ml) extract the aqueous layer, wash the combined organic layer with brine, Na 2 SO 4 Dry and remove the solvent under reduced pressure. Purification by silica gel column chromatography (PE:EA=1...
Embodiment 2
[1222] Example 2.3-(1-(3,3,3-trifluoropropionyl)piperidin-4-yl)benzo[d]oxazol-2(3H)-one (P2)
[1223]
[1224] Step 2.1. 3-(1-(3,3,3-trifluoropropionyl)piperidin-4-yl)benzo[d]oxazol-2(3H)-one (P2)
[1225]
[1226] To DCM (15ml) was added 3-(piperidin-4-yl)benzo[d]oxazol-2(3H)-one (1.5g, 6.88mmol) and K 2 CO 3 (1.99g, 13.8mmol), 3,3,3-trifluoropropyl chloride (1.05g, 11.4mmol) was added dropwise at 0°C. The reaction mixture was stirred at room temperature for 4 hours, the reaction mixture was filtered, and the filtrate was concentrated. The product was purified by silica gel column chromatography (PE:EA:DCM=1:1:1) to obtain the desired white solid (1.75 g, yield 77%). 1 H NMR (400MHz, CDCl 3 ):δ7.23(dd,J=5.2,1.2Hz,1H),7.21-7.12(m,2H),7.04(dd,J=8.0,1.2Hz,1H),4.94-4.89(m,1H), 4.44-4.37(m,1H),4.01-3.97(m,1H),3.38-3.27(m,3H),2.78-2.70(m,1H),2.37-2.23(m,2H),2.04-1.98(m ,2H).MS(ESI)m / z 329.1[M+H] + .
Embodiment 3
[1227] Example 3.3-(1-(2-(pyridin-4-yl)acetyl)piperidin-4-yl)benzo[d]oxazol-2(3H)-one (P3)
[1228]
[1229] Step 3.1. Synthesis of 3-(1-(2-(pyridin-4-yl)acetyl)piperidin-4-yl)benzo[d]oxazol-2(3H)-one (P3)
[1230]
[1231] In 3-(piperidin-4-yl)benzo[d]oxazol-2(3H)-one (1.5g, 6.88mmol) and K 2 CO 3 (2.8g, 20.6mmol) in DCM (20ml), 2-(pyridin-4-yl)acetyl chloride hydrochloride (2.0g, 10.3mmol) was added dropwise at 0°C. The reaction mixture was stirred at room temperature for 4 hours, the reaction mixture was filtered, and the filtrate was concentrated. Using high performance liquid chromatography (20-95% CH 3 CN aqueous solution) to obtain a white solid (1.07 g, yield 46%). 1 H NMR (400MHz, DMSO-d 6 ): δ8.51(d, J=6.0Hz, 2H), 7.35-7.29(m, 4H), 7.23-7.19(m, 1H), 7.15-7.11(m, 1H), 4.57(d, J=13.2 Hz, 1H), 4.43-4.35(m, 1H), 4.09(d, J=14.4Hz, 1H), 3.85(s, 2H), 3.23-3.16(m, 1H), 2.76-2.69(m, 1H) ,2.09-1.98(m,2H),1.86-1.81(m,2H).MS(ESI)m / z 338.0[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com