A stable polypeptide inhibitor derived from lsd1 substrate snail1 and its use
A technology for polypeptide inhibitors and uses, applied in the field of bioengineering, to achieve significant technological progress, increase treatment, and solve the effects of poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
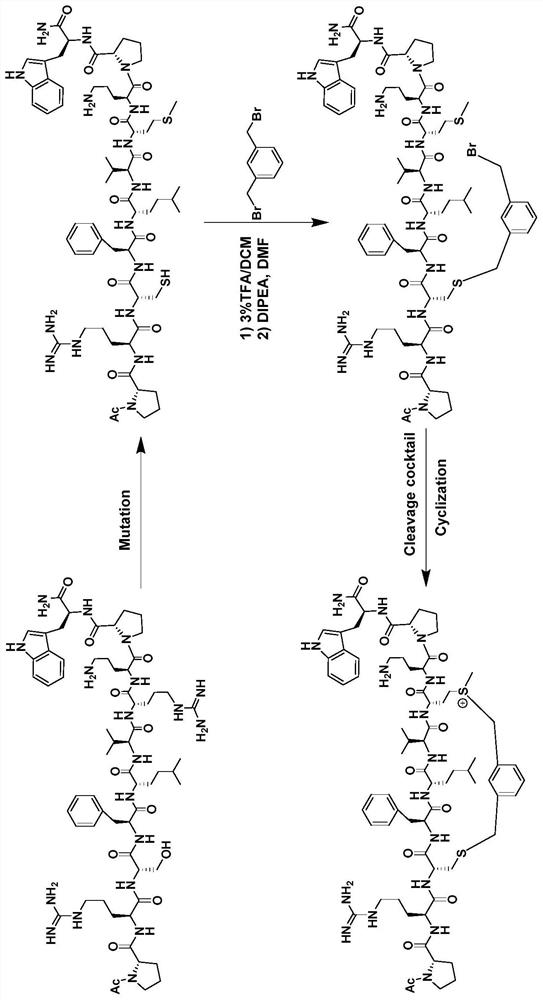
[0031] Embodiment 1: the design of polypeptide inhibitor
[0032] The present invention uses a chemical method based on sulfonium salt side chain stabilizing polypeptides to modify the polypeptide targeting LSD1. We select a section of polypeptide sequence (PRSFLVRKP) near the LSD1 selective substrate SNAIL1, because Arg7 of the polypeptide and Cys360 near the active pocket of LSD1 protease Appropriate for using the "sulfonium salt-based stabilization method" to stabilize the polypeptide and enable the covalent binding of the polypeptide as a ligand to the protein recognition without affecting the recognition of the target by the N-terminus of the polypeptide.
[0033] In order to further verify the importance of the peptide sequence, we screened a series of peptide sequences, such as Figure 4 As shown, there are polypeptide sequences of different lengths, sequences of the same length and different amino acid arrangements, and the inhibitory effect of these polypeptide sequen...
Embodiment 2
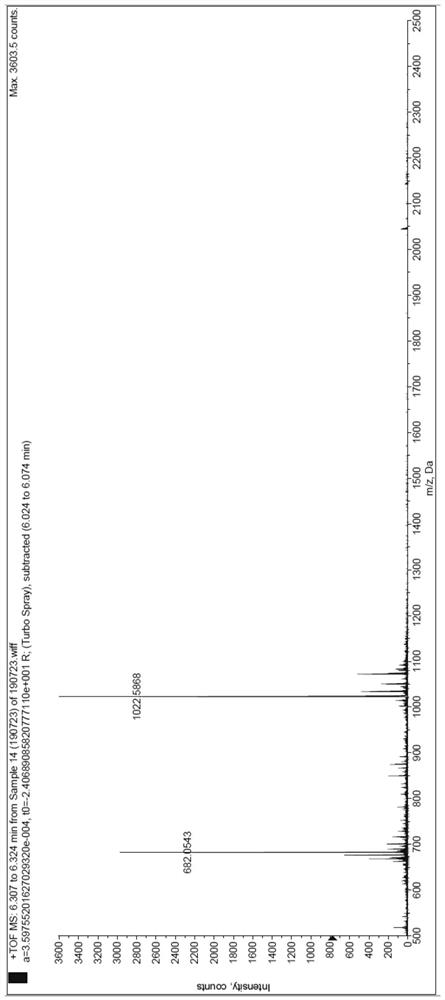
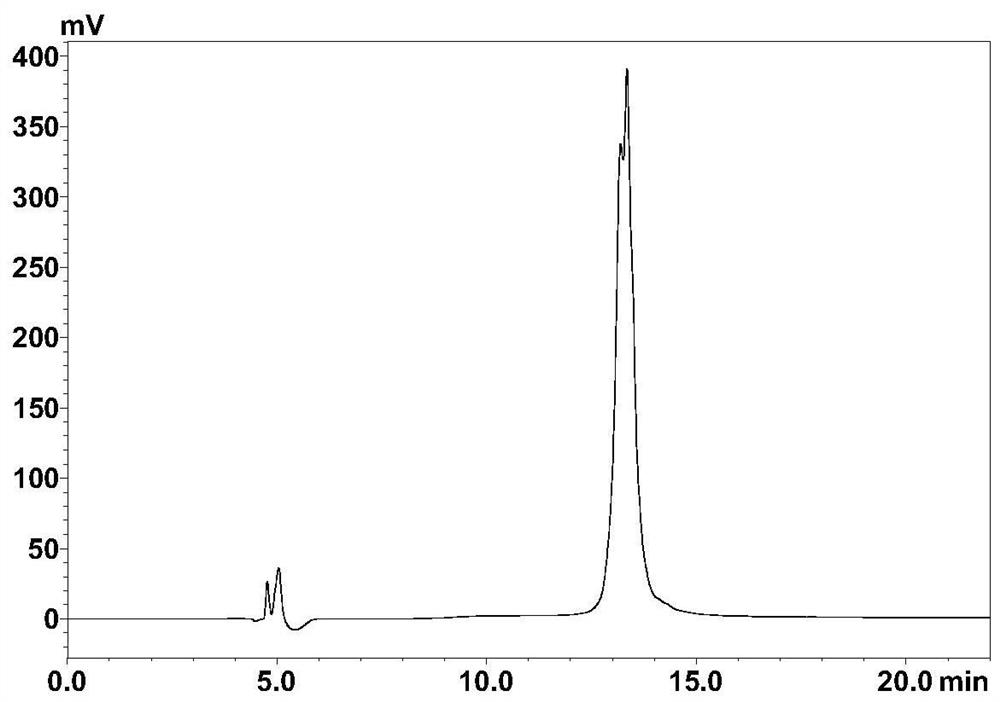
[0034] Embodiment 2: the synthetic preparation of polypeptide inhibitor
[0035] The solid-phase synthesis of polypeptides involved in the present invention all use Rink Amide MBHA resin, the resin with a loading degree of 0.37mmol / g is used for long peptides, and the resin with a loading degree of 0.54mmol / g is used for short peptides. The specific operation of solid-phase synthesis of peptides is as follows:
[0036] (1) Select Rink Amide MBHA resin with a suitable loading degree and swell with DMF for 30 minutes;
[0037](2) remove the Fmoc protecting group with 50% morpholine (dissolved in DMF), the reaction time is 30 minutes, repeat twice;
[0038] (3) Then use DMF (washing 4 times) and DCM (washing 4 times) to cross-wash respectively;
[0039] (4) Use 4 reaction equivalents of Fmoc-protected amino acids (obtained by conversion of the initial resin load), 3.9 equivalents of HCTU are mixed with DMF to dissolve, and then 8 equivalents of DIPEA are added for activation. T...
Embodiment 3
[0047] Example 3: Research on the Inhibitory Effect of Polypeptide LSD1 Enzyme Activity
[0048] The LSD1-HRP coupling reaction can be used to detect LSD1 activity. The principle is that the by-product H is produced in the process of substrate demethylation catalyzed by LSD1. 2 o 2 , so horseradish peroxidase (HRP) can be used to catalyze H 2 o 2 React with Amplex Red (a dye) to produce Resorufin (a substance that can produce strong fluorescence), and the enzymatic activity of LSD1 can be indirectly obtained by detecting the fluorescence intensity of the product. in the H 2 o 2 is reduced to H 2 In the process of O, Amplex Red acts as an electron donor, which is oxidized into Resorufin for detection of fluorescence. Its maximum excitation wavelength is about 530nm, and its maximum emission wavelength is about 590nm, and the reactants Amplex Red and H 2 o 2 There is no absorption at 530nm and will not interfere with detection. According to the limitations of experimen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com