Use of nitazoxanide and pharmaceutically acceptable salt thereof in preparation of drug for treating bladder cancers
A technology for nitazoxanide and bladder cancer, which is applied in the new medical field of nitazoxanide, can solve the problems of poor curative effect, large toxic and side effects, and achieves inhibition of dryness maintenance, inhibition of bladder tumor growth, good treatment and application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
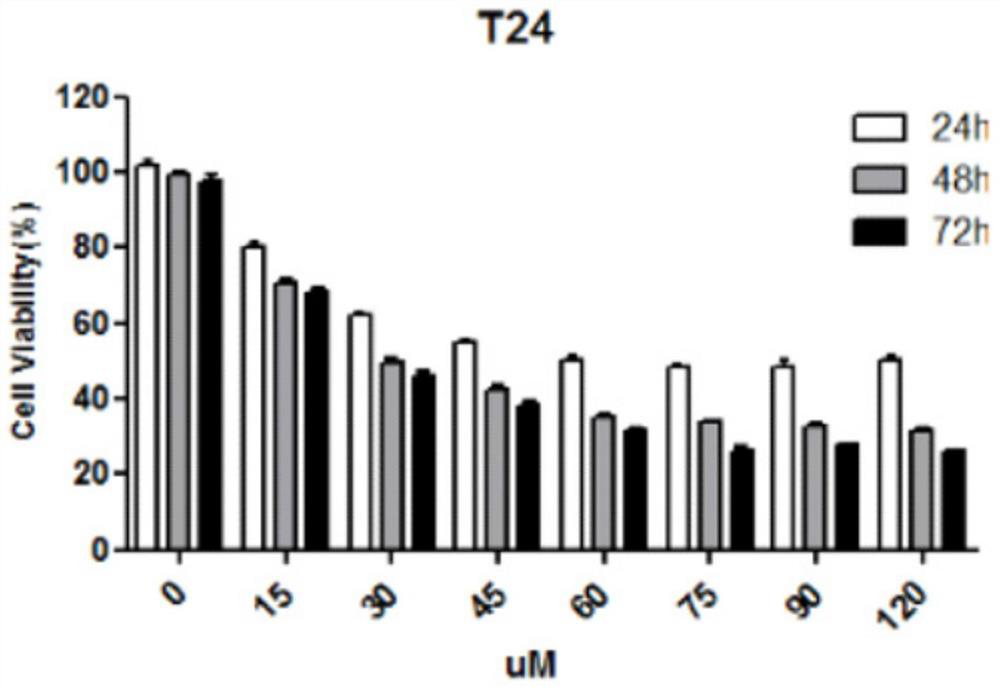
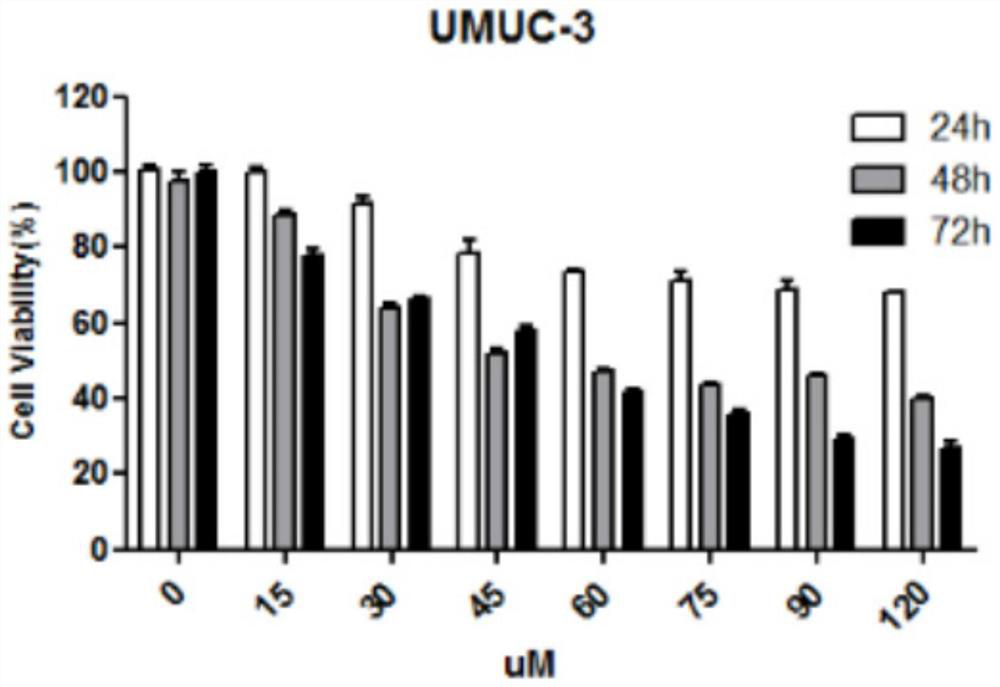
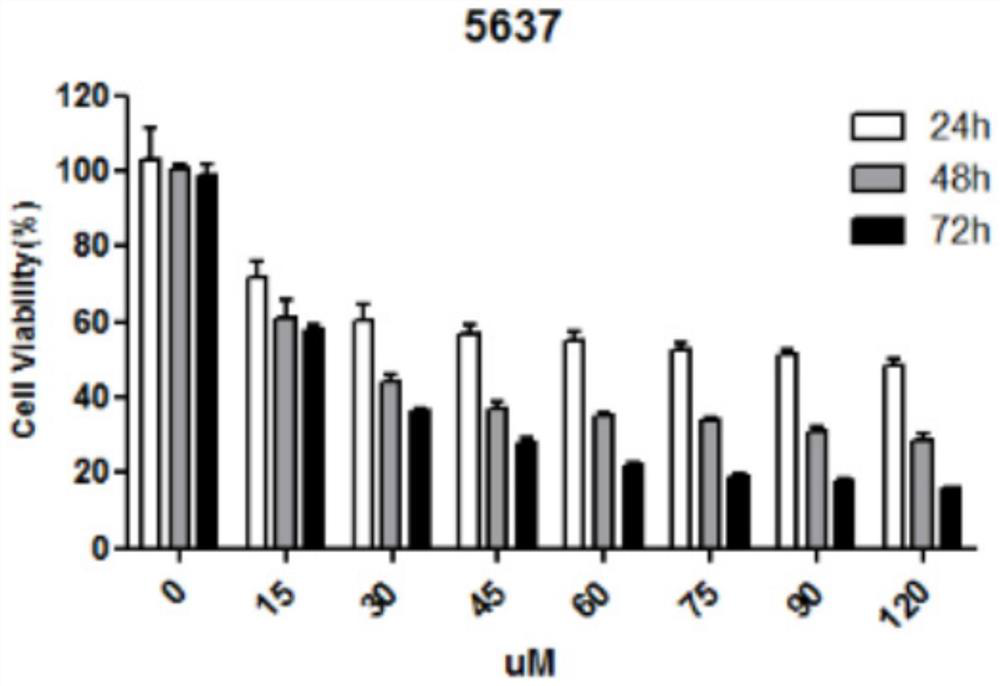
[0034] Detection of the killing effect of nitazoxanide on bladder cancer cells
[0035] 1) Bladder tumor cell culture in vitro: Bladder cancer cells used in this experiment mainly include UMUC-3, T24, 5637, and MGHU3 cells, among which UMUC-3 and T24 cells were treated with DMEM containing 10% fetal bovine serum and 1% double antibody Culture medium, 5637 and MGHU3 cells use RPMI1640 culture medium containing 10% fetal bovine serum and 1% double antibody, all at 37°C, 5% CO 2 cultured in an incubator. When the cells grow to 80% density, trypsinize, collect the cells by centrifugation and resuspend in the culture medium, take an appropriate amount of cell suspension for passage.
[0036] 2) In vitro proliferation inhibition experiment: take a certain number of cells (0.6×10 4 Each well) was inoculated in a 96-well plate, and a control group and an experimental group were set up, with 6 replicate wells in each group. After 12 hours of culture, the above-mentioned bladder tumor...
Embodiment 2
[0041] Detection of the damage of nitazoxanide to the mitochondria of bladder cancer cells
[0042] 1), JC-1 staining to detect changes in cell membrane potential (MMP): take a certain number of cells (4×10 5 / well) inoculated in 6-well plates, set up control and experimental groups, treated MGHU3 cells with 60 μM concentration of nitazoxanide after 12 hours of culture, continued to culture for 6, 12, and 24 hours; digested and collected cells and washed them with pre-cooled PBS, using a concentration of Cells were resuspended in 5 μM JC-1 solution (PBS configuration), and stained at 37°C for 15 minutes in the dark; after filtering through a 300-mesh sieve, the changes of mitochondrial membrane potential MMP were detected by Guava easyCyte flow cytometer (JC-1 was Polymer state: Ex=488nm, Em=575nm, JC-1 is monomeric state: Ex=488nm, Em=525nm). The data were analyzed using FlowJo software, and the results were as follows: image 3 shown.
[0043] The change of mitochondrial ...
Embodiment 3
[0047] Detection of Bladder Cancer Cell Apoptosis Induced by Nitazoxanide
[0048] 1), Annexin V-FITC / PI double staining method to detect cell apoptosis: take a certain number of cells (4 × 10 5 Each well) was inoculated in a 6-well plate, and the control group and the experimental group were set up. After 12 hours of culture, the MGHU3 cells were treated with different concentrations of nitazoxanide, and the culture continued for 48 hours; The cells were resuspended with 3 μL of PI in PBS solution (500 μL), and stained at 37°C for 15 minutes in the dark; after filtering through a 300-mesh sieve, the cell apoptosis was detected with a Guava easyCyte flow cytometer (among them, Annexin V-FITC : Ex=488nm, Em=525nm, PI: Ex=488nm, Em=620nm). The data were analyzed using FlowJo software, and the results were as follows: Figure 5 shown.
[0049] from Figure 5 It can be seen that after Annexin V-FITC / PI double staining, compared with the control group, early apoptosis occurred ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com