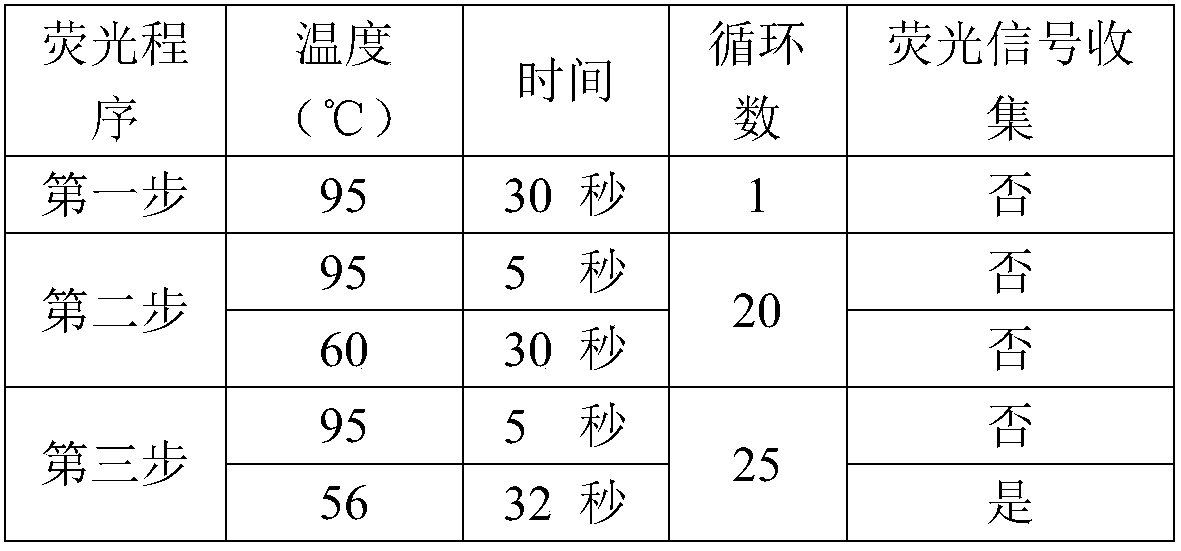
Patents
Literature
308 results about "Urinary Bladder Cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
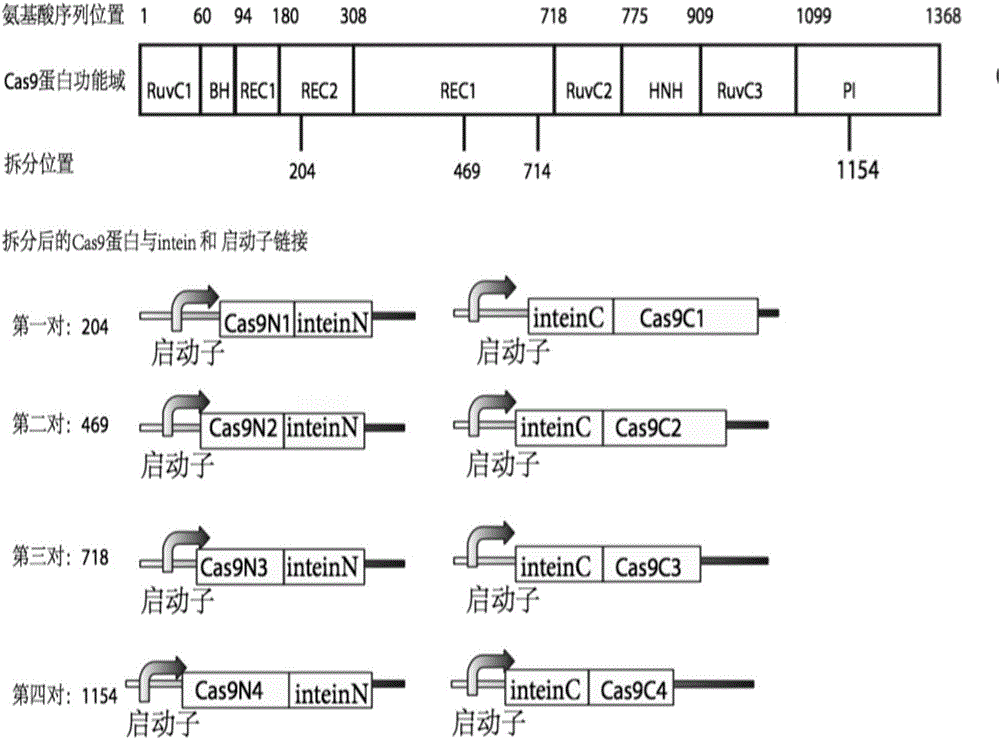
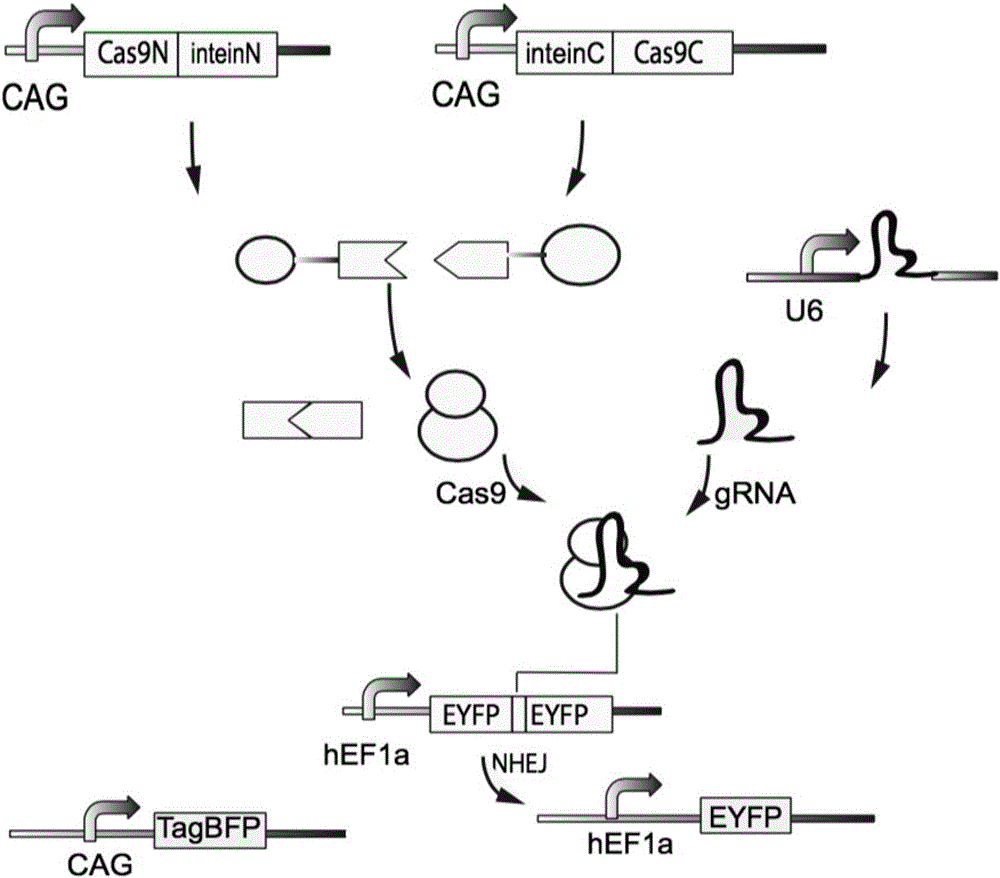
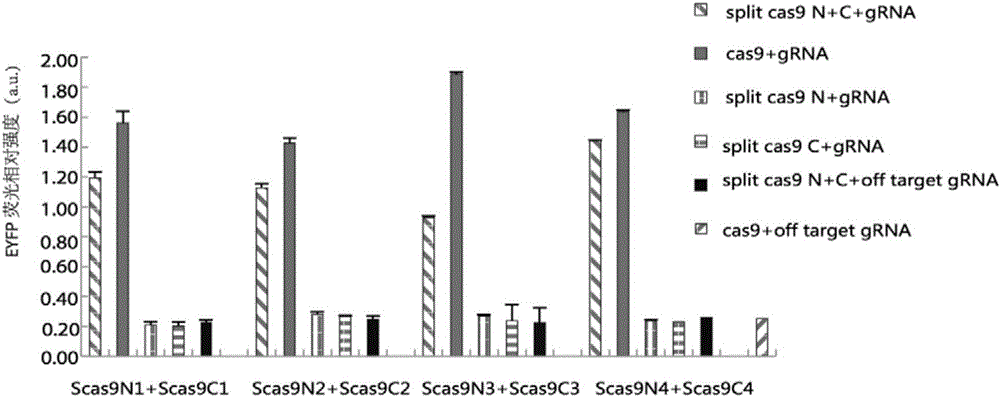
Method for carrying out gene editing and expression regulation by utilizing Cas splitting system
Owner:TSINGHUA UNIV
Apoptosis inducing adamantyl derivatives and their usage as anti-cancer agents
InactiveUS6127415APreventing and controlling photoinducedPreventing and controlling and chronologic agingBiocideCosmetic preparationsDiseaseAnticarcinogen
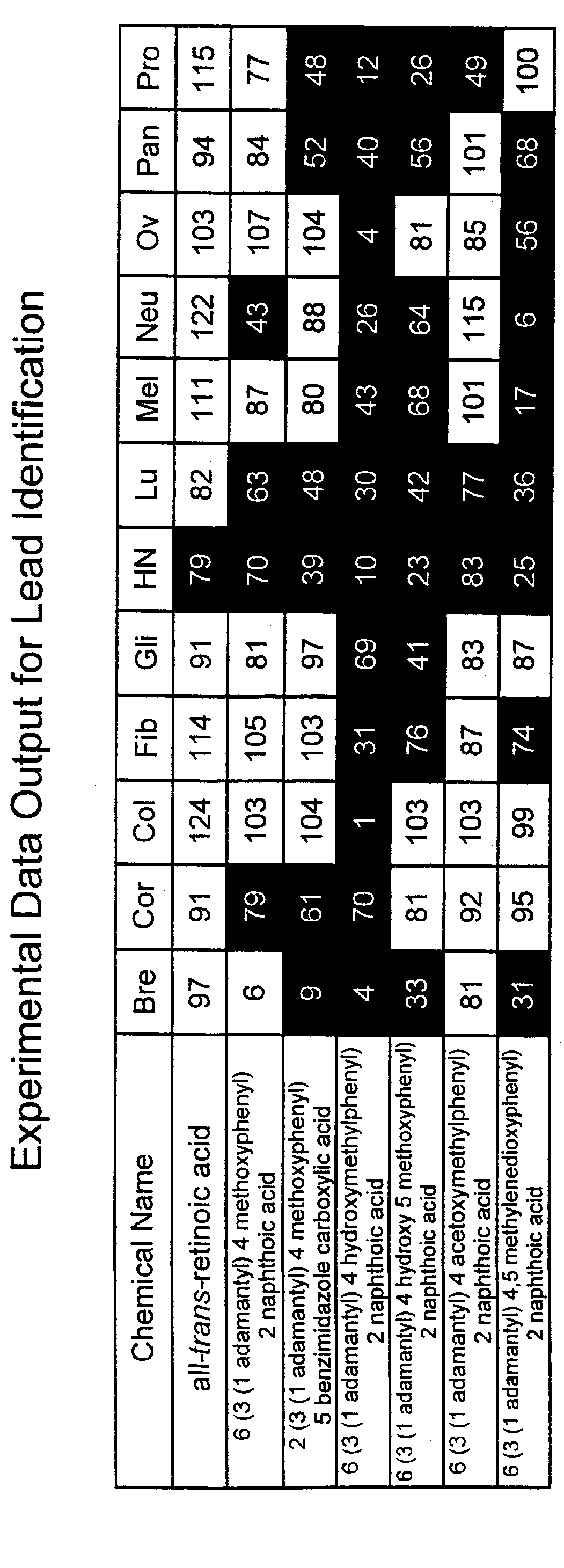
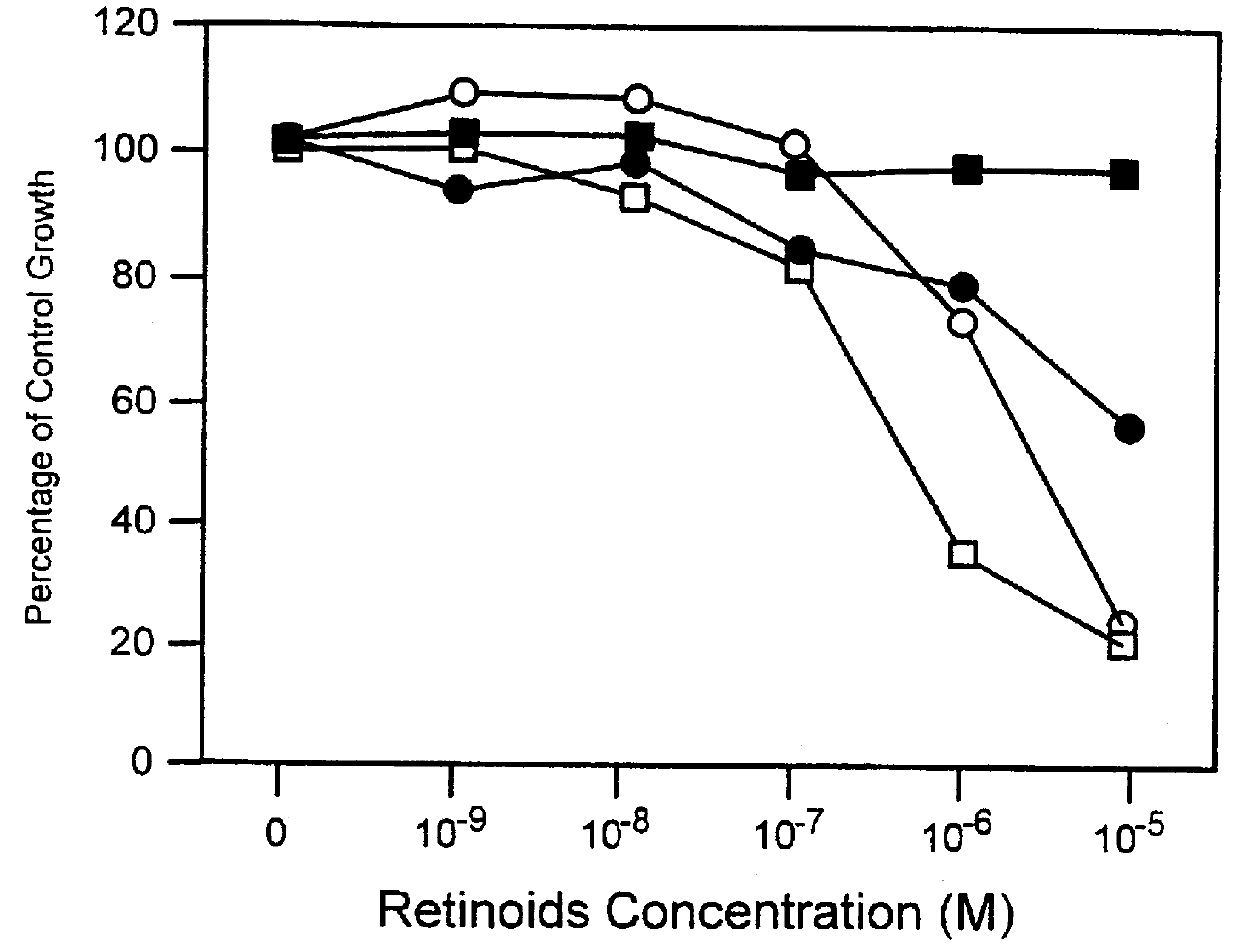
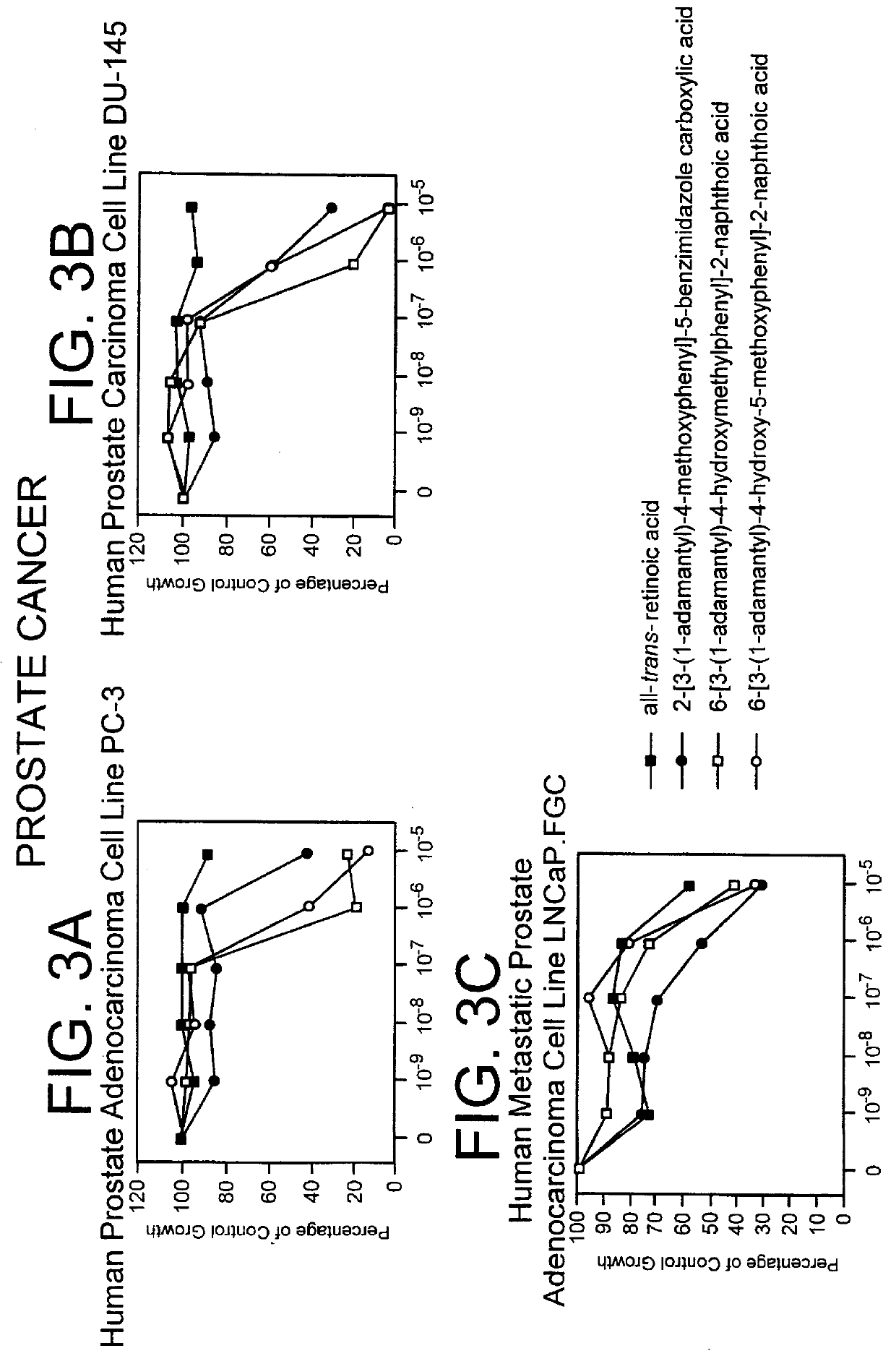
PCT No. PCT / US97 / 11564 Sec. 371 Date Apr. 14, 1999 Sec. 102(e) Date Apr. 14, 1999 PCT Filed Jul. 8, 1997 PCT Pub. No. WO98 / 01132 PCT Pub. Date Jan. 15, 1998The present invention relates to specific adamantyl or adamantyl group derivative containing retinoid compounds induce apoptosis of cancer cells. These adamantyl retinoid derivatives are useful for the treatment of many cancers and solid tumors, especially androgen-independent prostate cancer, skin cancer, pancreatic carcinomas, colon cancer, melanoma, ovarian cancer, liver cancer, small cell lung carcinoma, non-small cell lung carcinoma, cervical carcinoma, brain cancer, bladder cancer, breast cancer, neuroblastoma / glioblastoma, and leukemia. Also, the invention relates to novel adamantyl or adamantyl group derivative compounds which are useful as active agents for the treatment or prevention of keratinization disorders and other dermatological conditions, and other diseases.
Owner:GALDERMA RES & DEV SNC
Compositions and methods for the enhanced uptake of therapeutic agents through the bladder epithelium
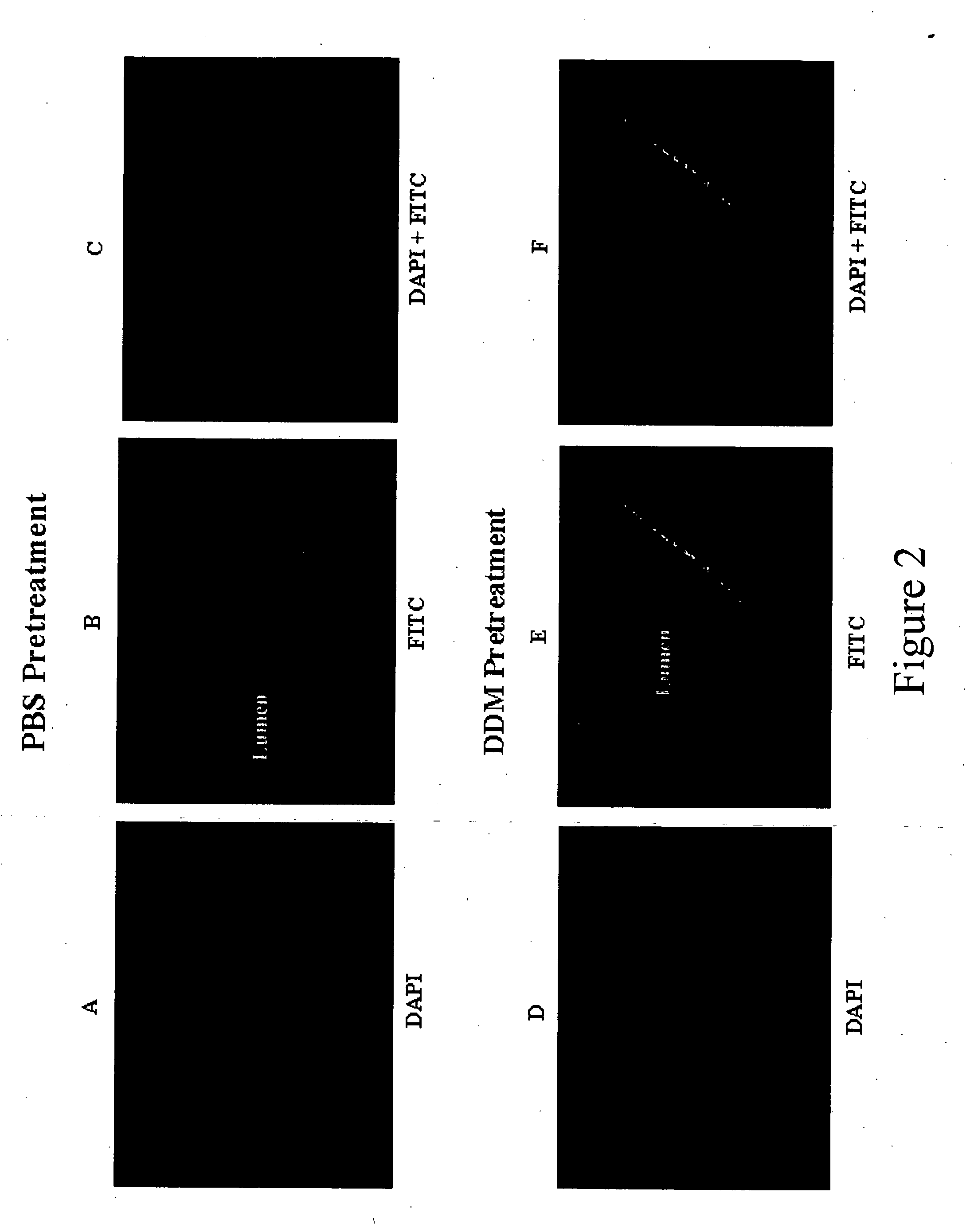
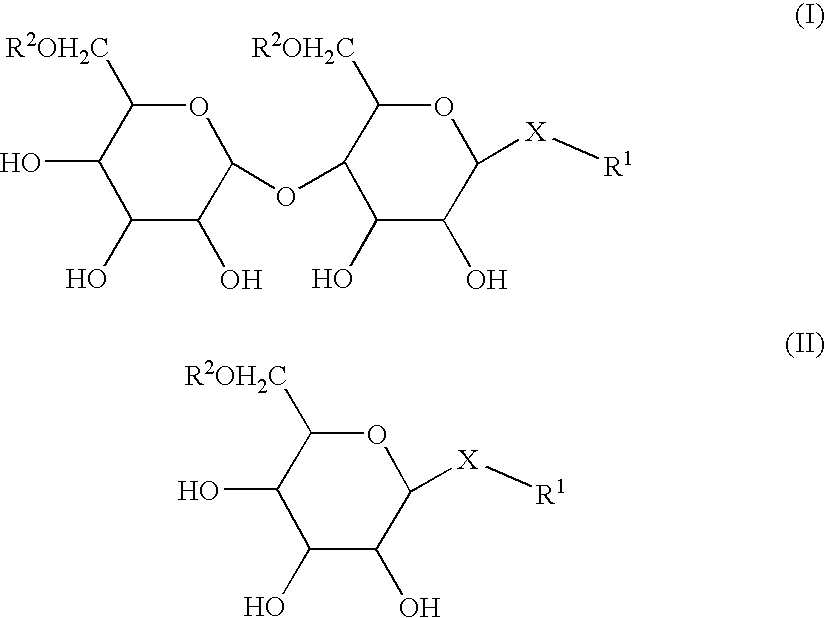
InactiveUS20050059613A1Good water solubilityGood curative effectBiocideCarbohydrate active ingredientsEpitheliumBladder cancer
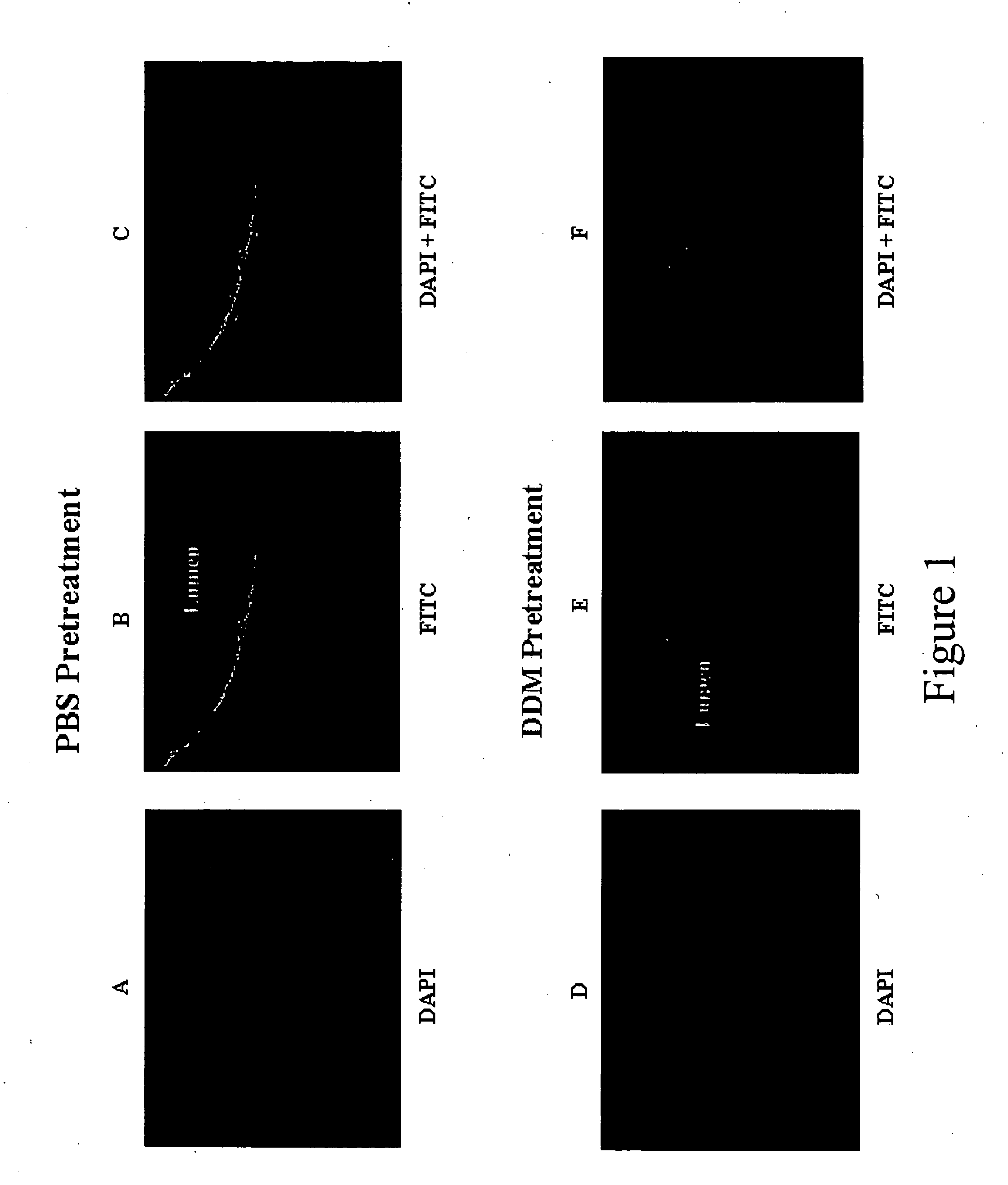
Compositions and methods for enhancing introduction of therapeutic agents into the bladder epithelium for the treatment of bladder diseases and disorders such as bladder cancer are described. According to one method, the luminal surface of the bladder is contacted with a composition comprising a bladder enhancer and a therapeutic agent for the treatment of the bladder disease. According to an alternative method, the luminal surface of the bladder is first contacted with a pretreatment composition comprising a bladder enhancer and subsequently contacted with a composition comprising a therapeutic agent for the treatment of the bladder disease. The transduction enhancing agent can be a mono-, di-, or poly-saccharide having a lipophilic substituent such as n-dodecyl-β-D-maltoside (DDM). Compositions comprising a transduction enhancing agent and a therapeutic agent for the treatment of a bladder disease are also described.
Owner:CELLS GENESYS INC
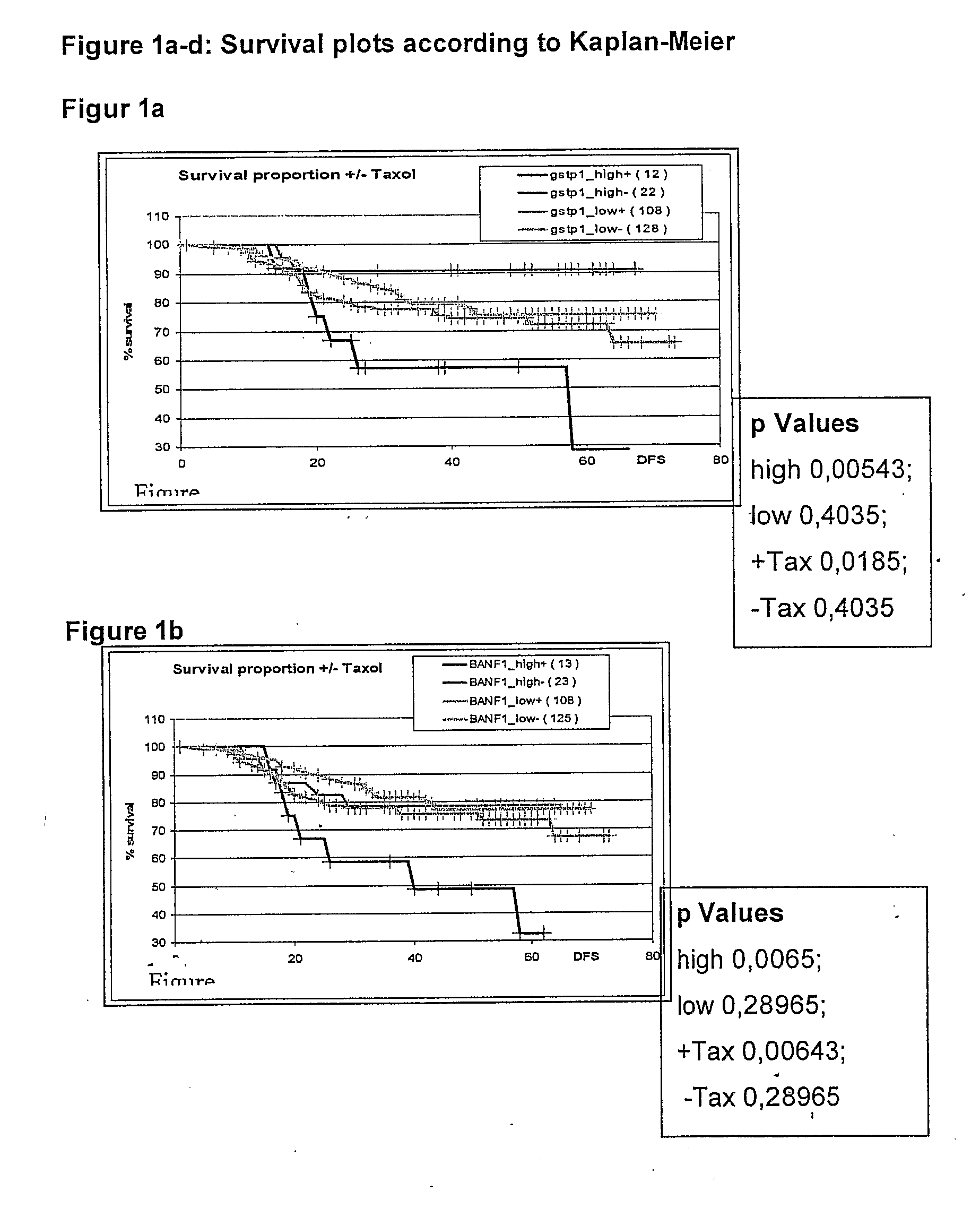
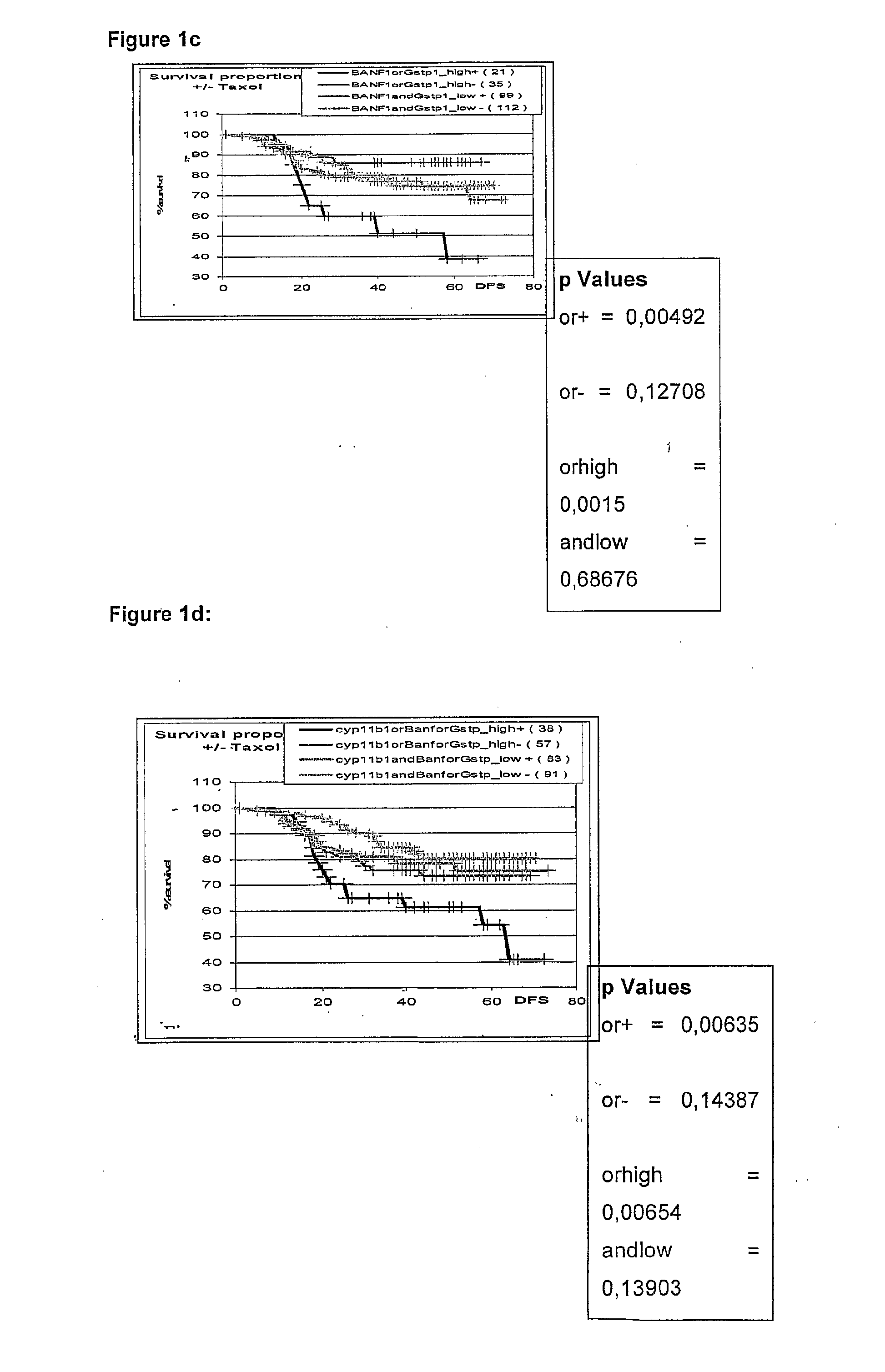
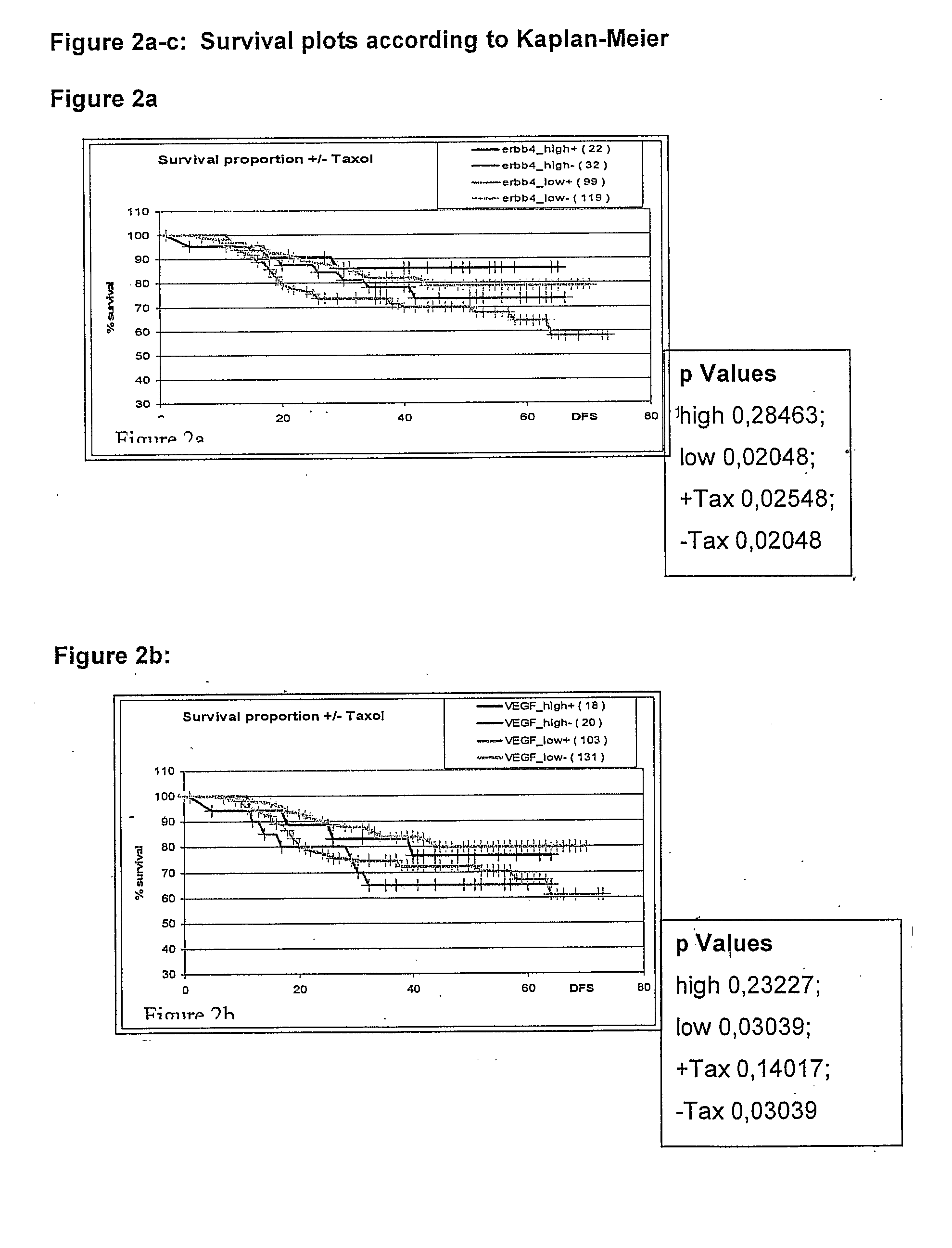
Genetic Alterations Useful For The Response Prediction of Malignant Neoplasia to Taxane-Based Medical Treatments
The invention provides novel compositions, methods and uses, for the diagnosis, prognosis, prediction, prevention and aid in treatment of malignant neoplasia such as breast cancer, ovarian cancer, gastric cancer, colon cancer, esophageal cancer, mesenchymal cancer, bladder cancer or non-small cell lung cancer. Genes that are chromosomally amplified in breast tissue of breast cancer patients are disclosed. Further disclosed are chromosomally amplified genes and non-amplified genes that correlate to Taxane resistance, Taxane benefit or adverse Taxane reaction, which can be used as an aid to make therapy dicisions.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS GMBH
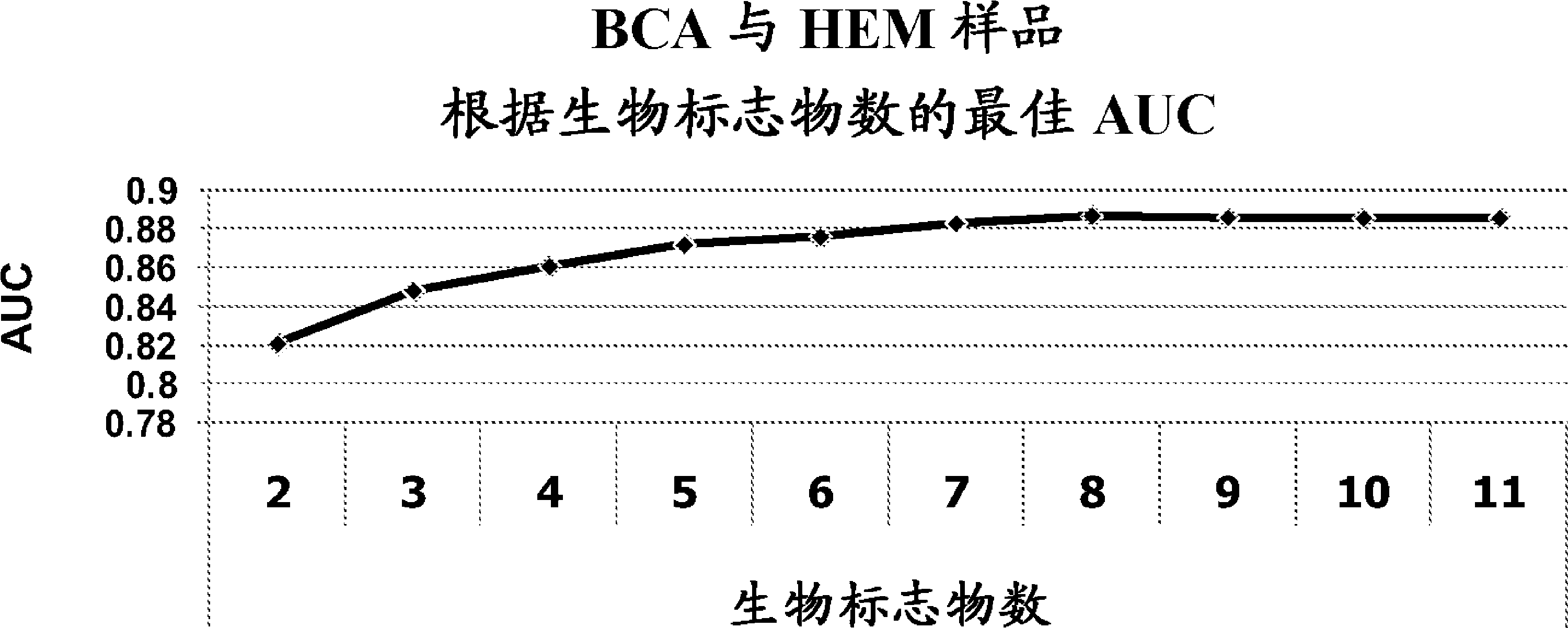
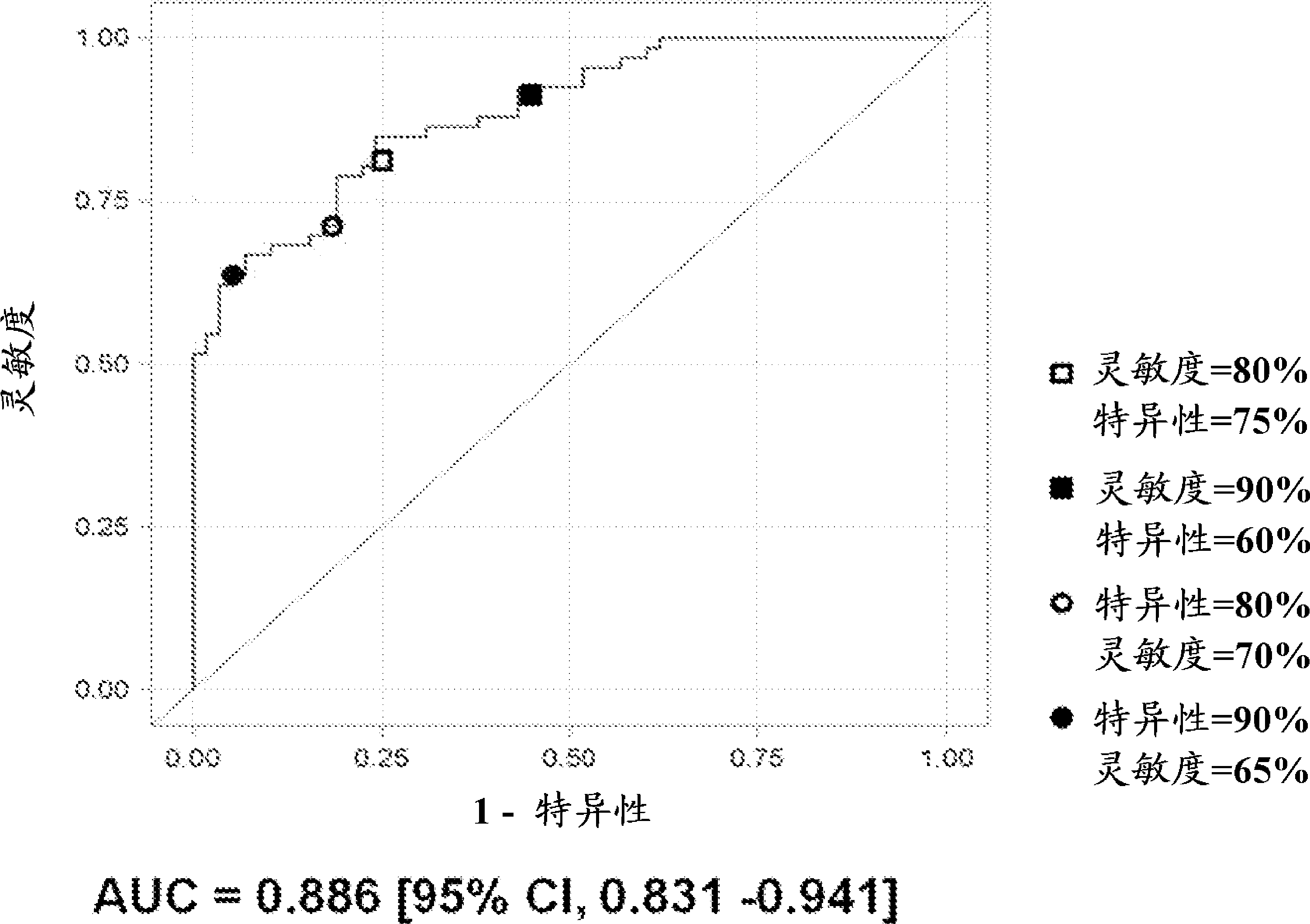
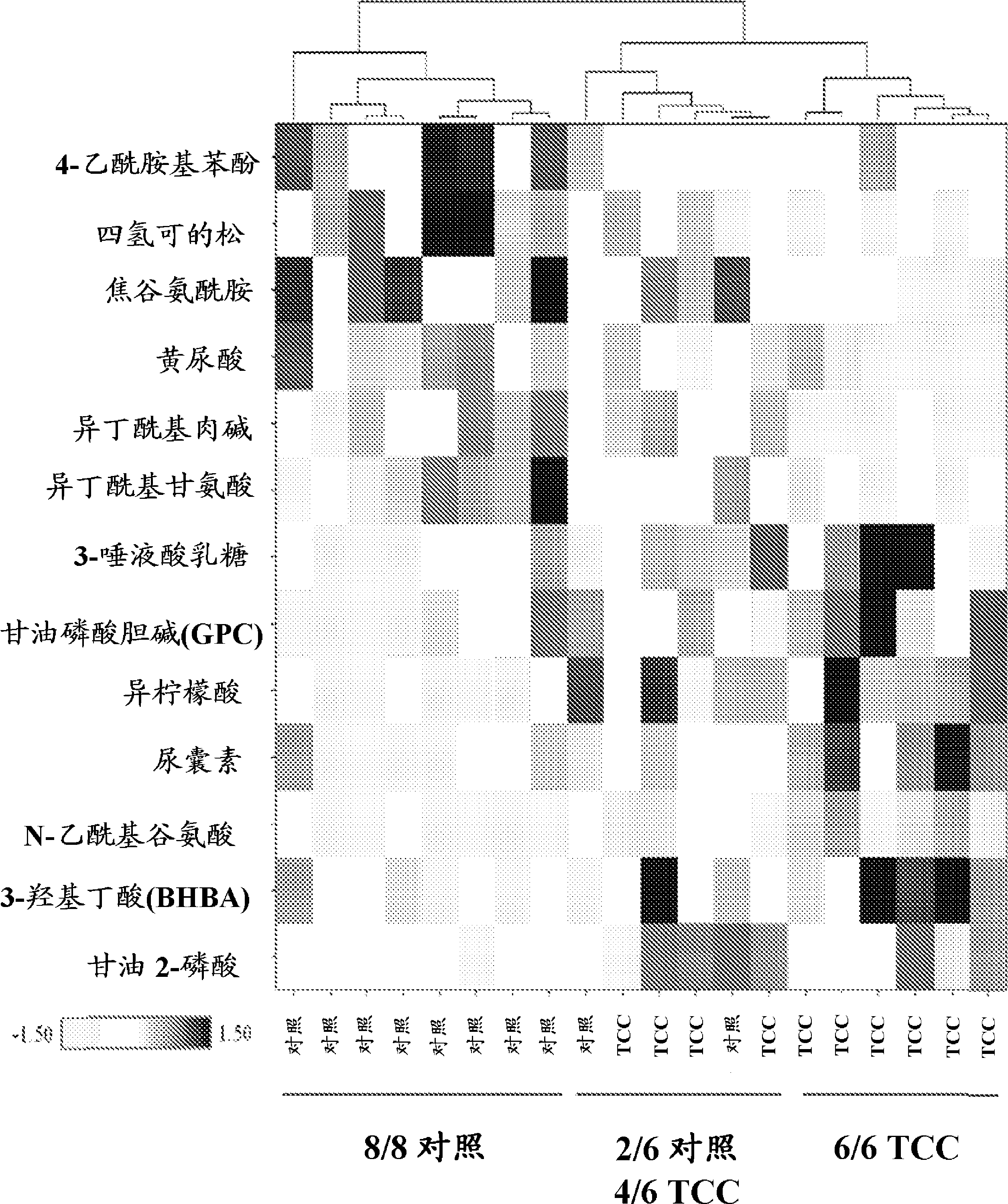
Bladder cancer patient urine specific metabolite spectrum, establishing method and application
The invention provides a bladder cancer patient urine specific metabolite spectrum, an establishing method and an application, and especially provides a method for establishing a spectrum of specific metabolites in urine of bladder cancer patients, and a method for screening a related specific biomarker. Through metabonomics, especially metabonomics based on liquid chromatography-mass spectrometry combined technology, the invention establishes a bladder cancer patient urine specific metabolite spectrum. The invention provides a basis for early diagnosis of bladder cancer and bladder cancer diseases with different pathological stages, and also provides a spectrum of specific metabolites in urine of bladder cancer patients and a related specific biomarker. The method provided by the invention has the characteristics of noninvasiveness, convenience, and rapidness, can accurately reflect the difference of metabolite spectra of bladder cancer patients and normal people, and has high specificity.
Owner:BGI GENOMICS CO LTD
Method and reagent kit for analyzing and diagnosing bladder cancer by means of uropsammus DNA methylation profile
The invention relates to a method used for detecting whether a detected object suffers from bladder cancer, comprising the following steps: (a) the detected object provides a urinary sediment sample; (b) the methylation spectrum formula of a specific sequence (named as a gene in the following) in one or a plurality of gene promoter areas CpG in the sample is measured; and (c) the methylation spectrum formula of the detected object is compared with that of a normal object, if one or a plurality of genes are in high methylation state, the detected object is proved to suffer from the bladder cancer. The invention also provides a set of kit used for diagnosing the bladder cancer.
Owner:SHANGHAI INST OF ONCOLOGY
Biomarkers for bladder cancer and methods using the same
Methods for identifying and evaluating biochemical entities useful as biomarkers for bladder cancer, target identification / validation, and monitoring of drug efficacy are provided. Also provided are suites of small molecule entities as biomarkers for bladder cancer.
Owner:METABOLON
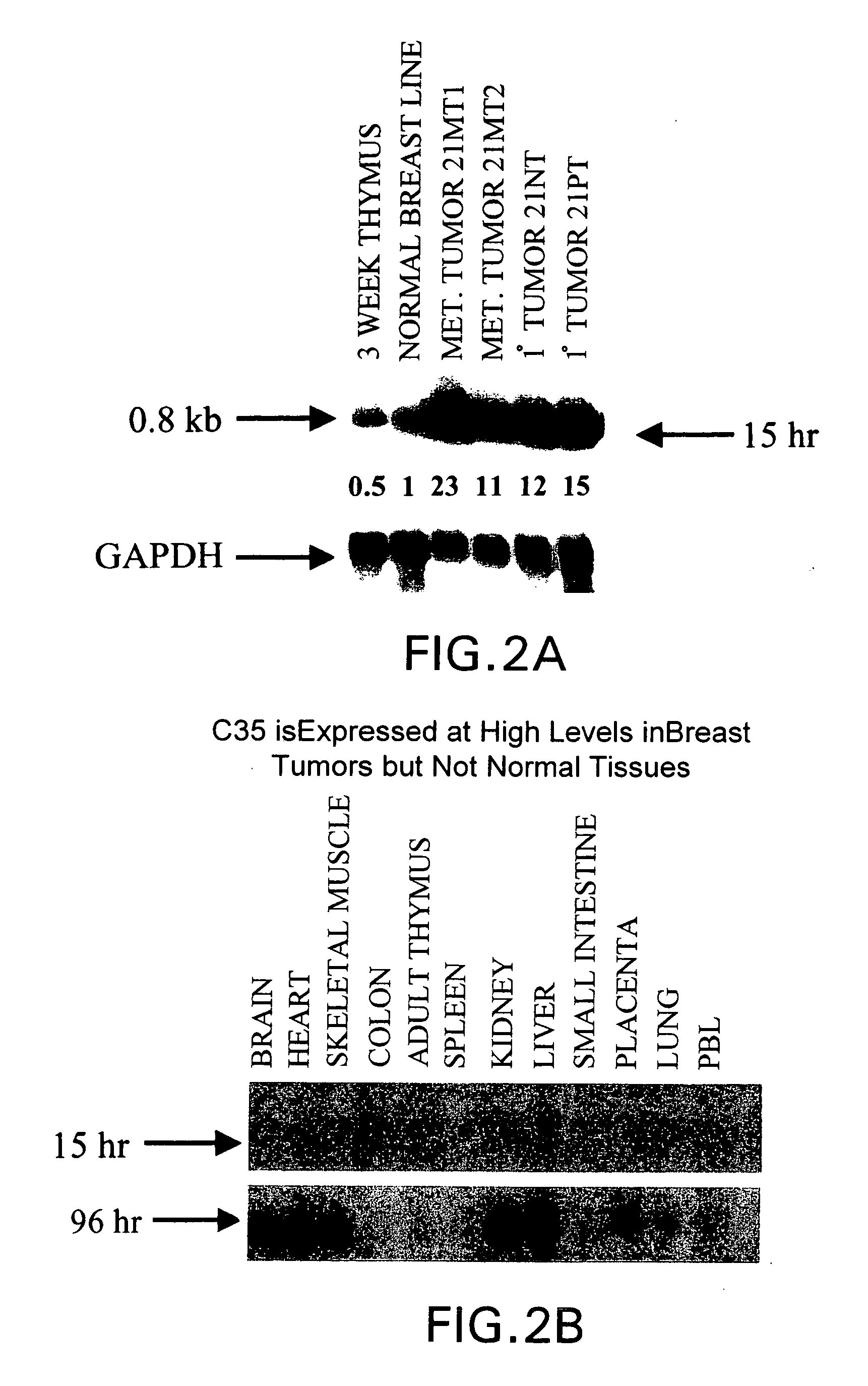
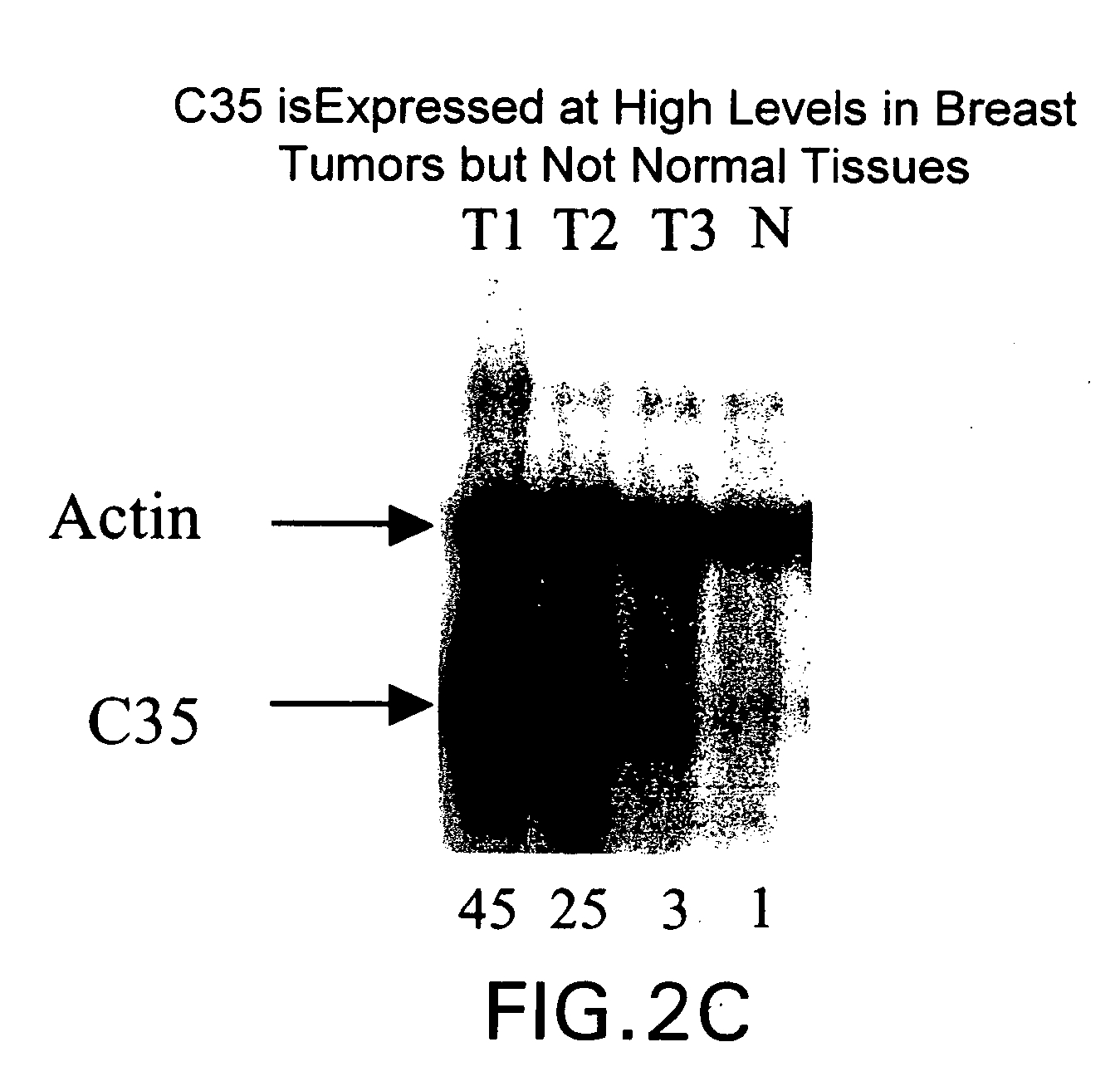
Gene differentially expressed in breast and bladder cancer, and encoded polypeptides
The present invention is directed to antibodies or antigen binding fragments thereof that specifically bind to the cancer specific antigen, C35. This invention also relates to a polynucleotide encoding the antibody or antigen binding fragment thereof as well as vectors and host cells comprising the polynucleotide. The present invention further relates to a composition comprising the antibody or antigen binding fragment thereof or further comprising a chemotherapeutic agent, specifically taxol. The invention is also directed to therapeutic and diagnostic methods of using antibodies against C35, preferably more than one anti-C35 antibody in combination with a chemotherapeutic agent, to treat C35 associated cancer.
Owner:UNIVERSITY OF ROCHESTER
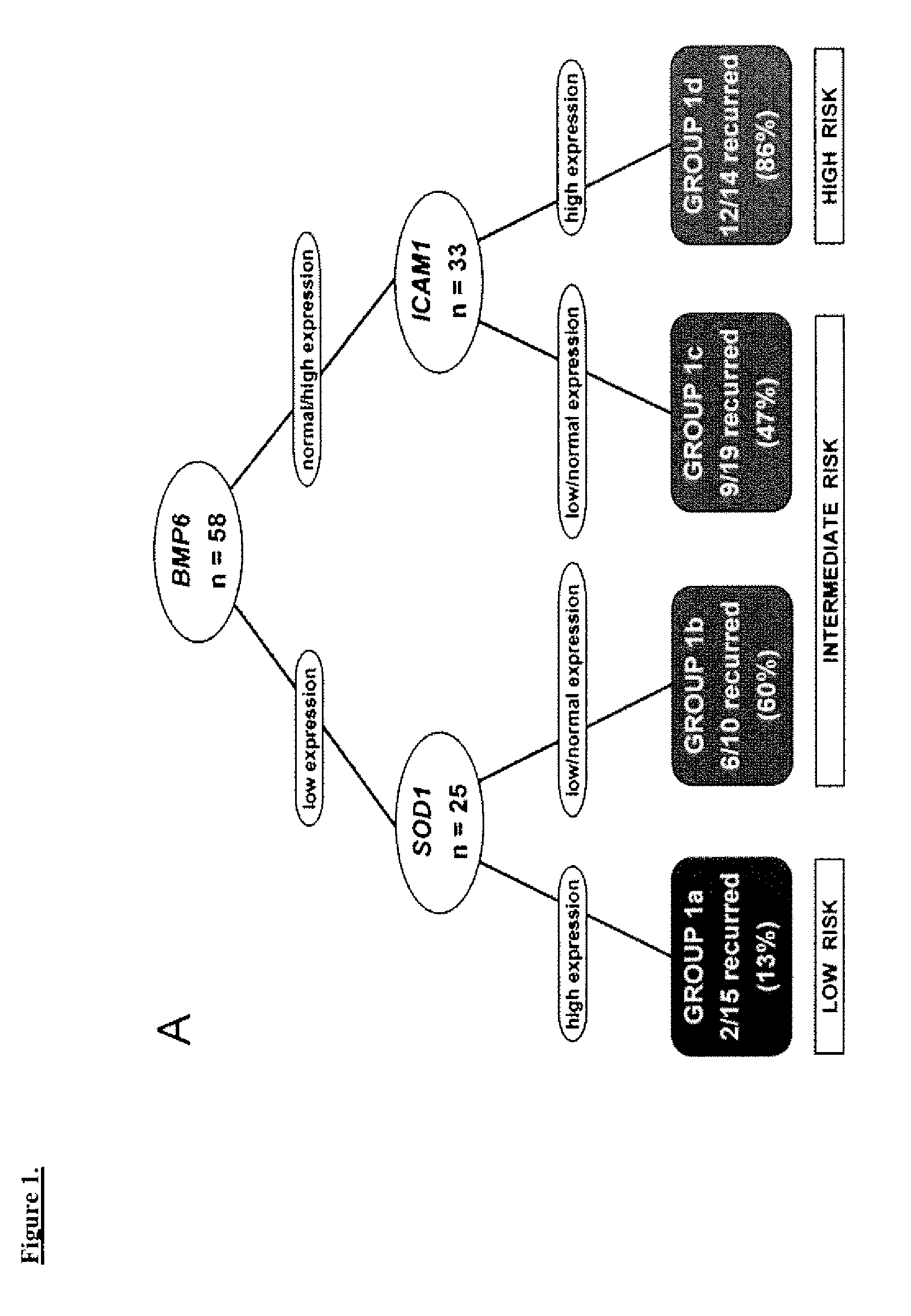
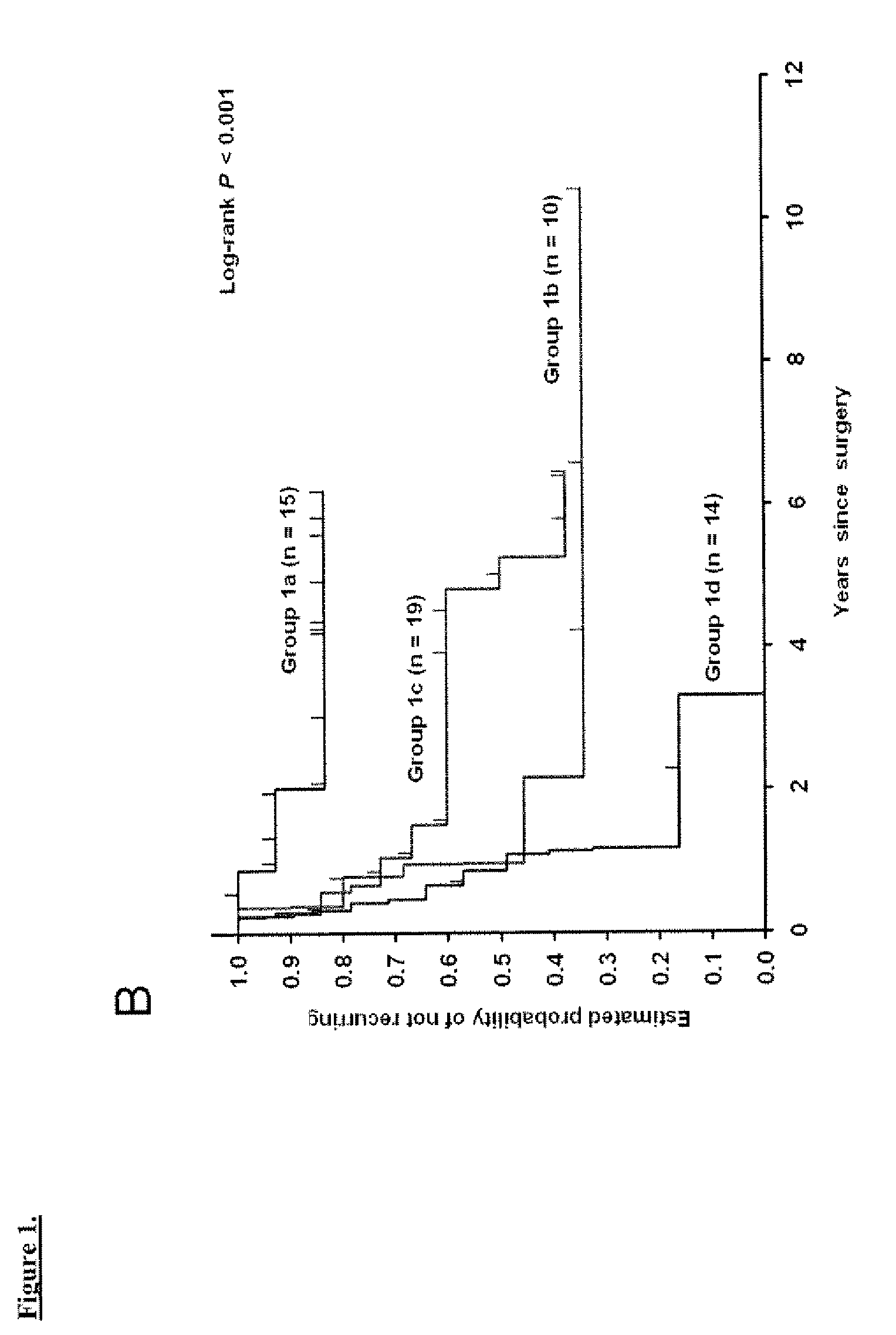
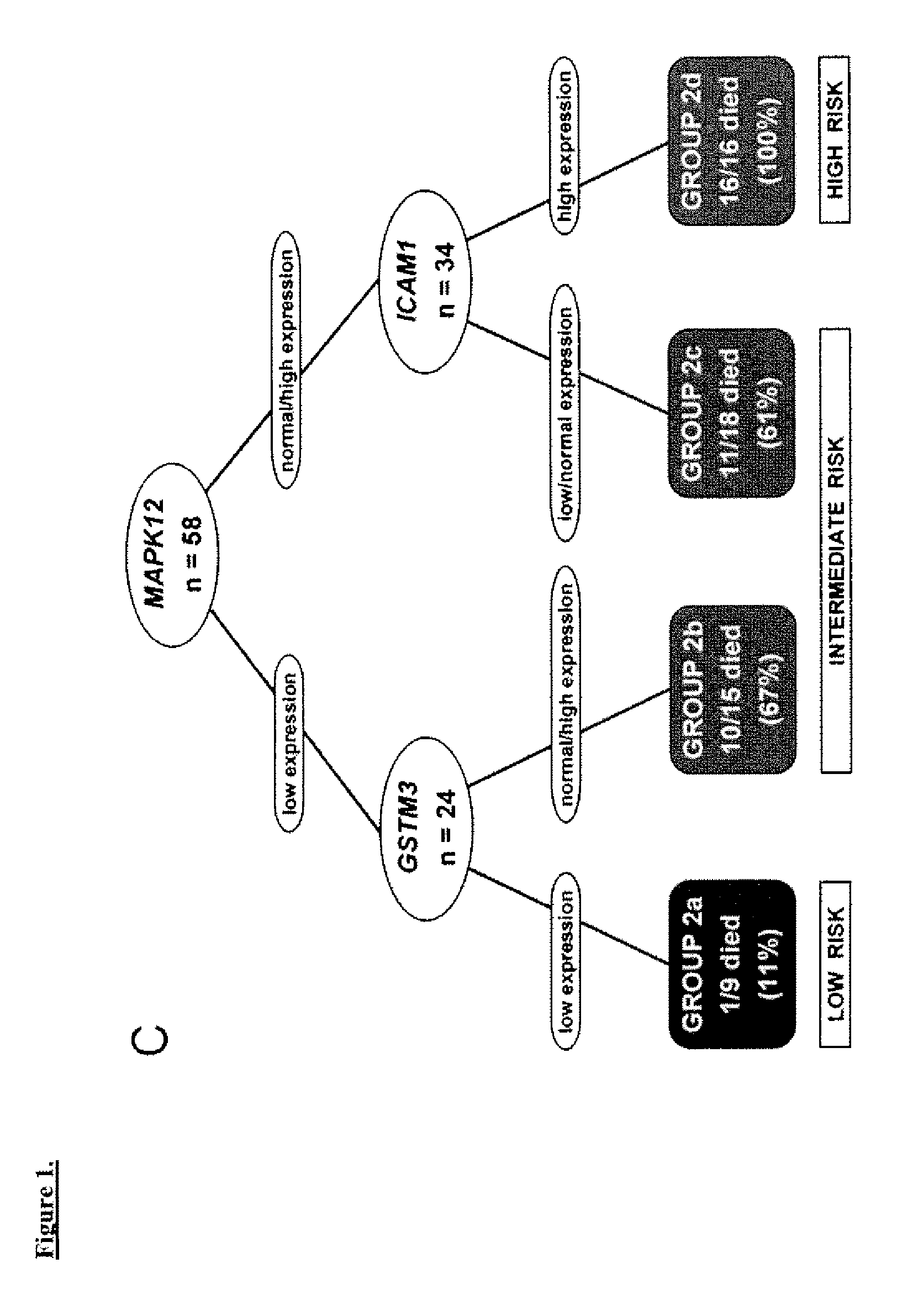
Prognostic panel for urinary bladder cancer
The present invention relates to methods of prognosing urothelial carcinoma. In one embodiment, the present invention provides a method of prognosing urothelial carcinoma by determining expression levels of JUN, MAP2K6, STAT3 and / or ICAM1. In another embodiment, the present invention provides an single prognostic panel made up of eight gene markers. In another embodiment, the present invention provides a single prognostic panel made up of eleven gene markers.
Owner:UNIV OF SOUTHERN CALIFORNIA
Methods for diagnosing solid tumors
InactiveUS20130129681A1Peptide/protein ingredientsMicrobiological testing/measurementTherapeutic treatmentWilms' tumor
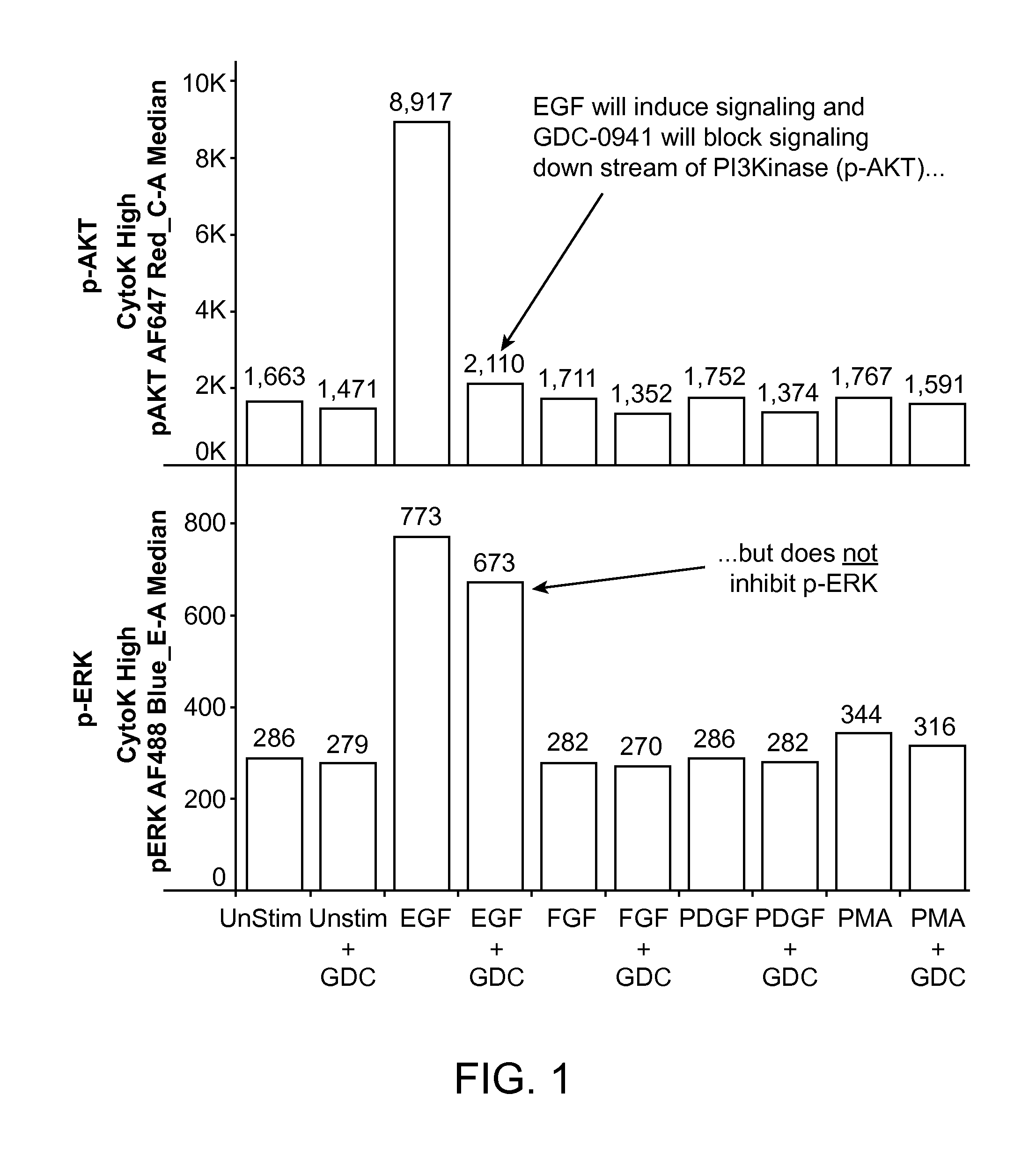
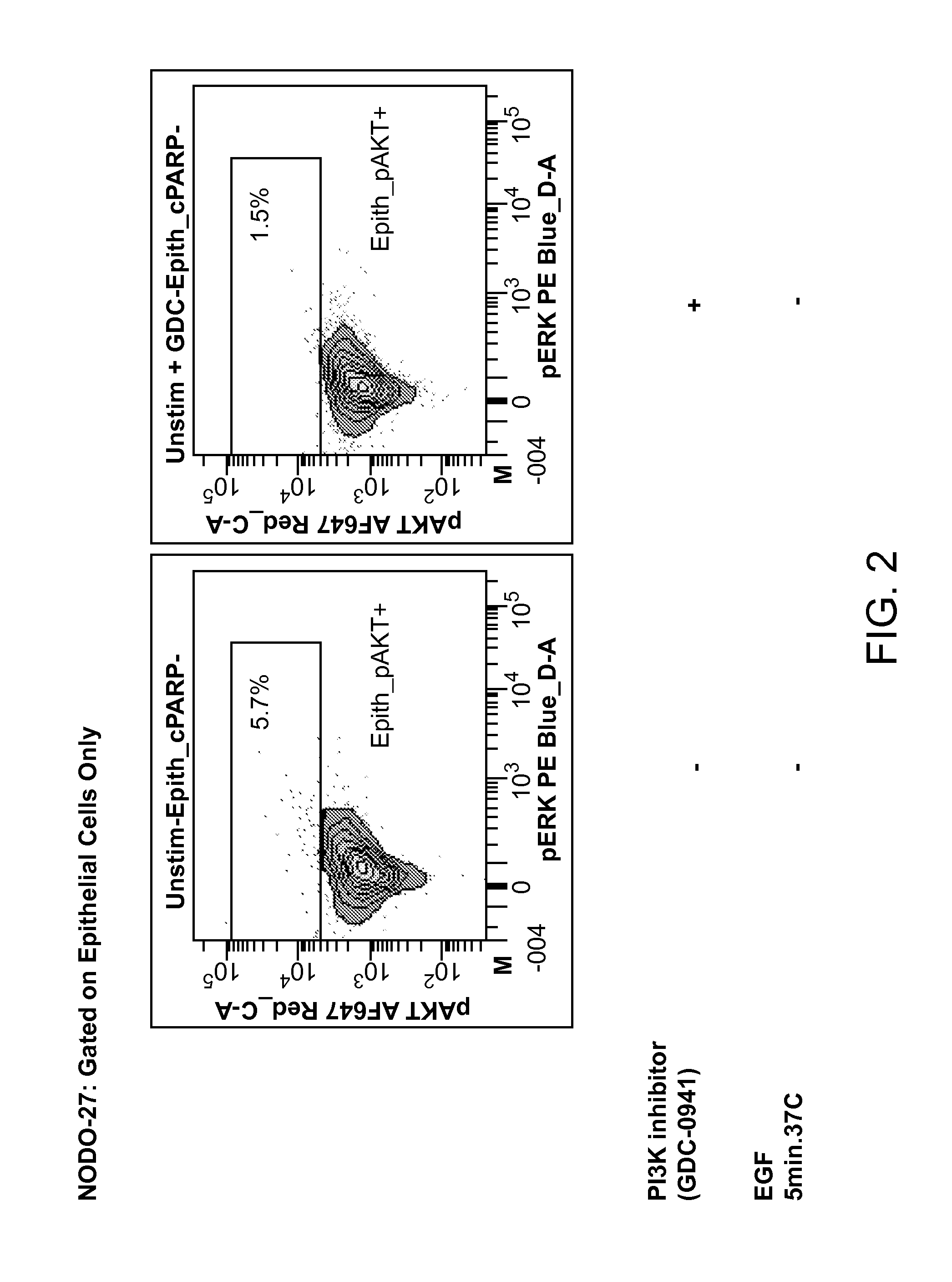
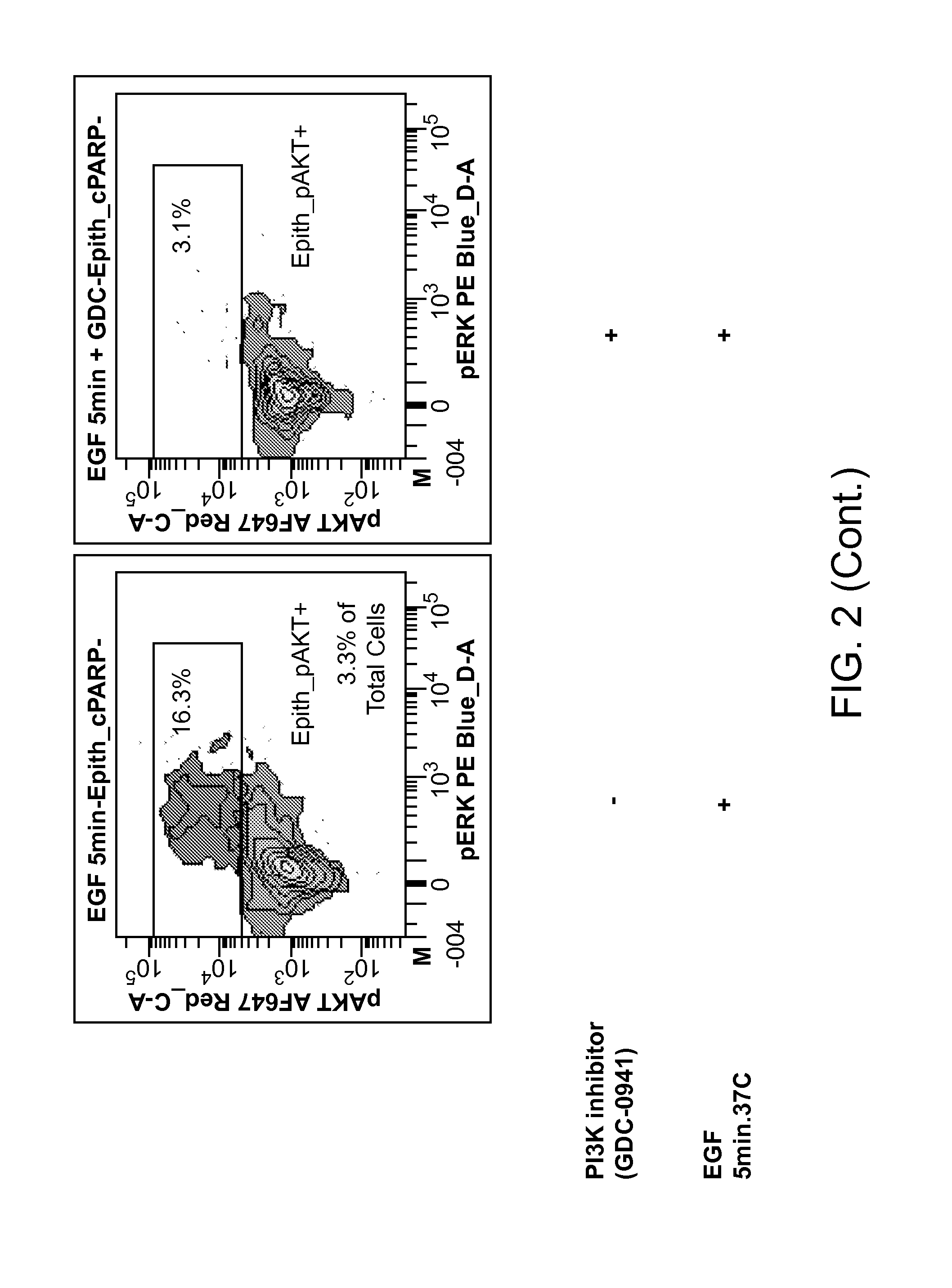
An embodiment of the present invention is useful for identifying tumor cells in bladder cancer washes, pleural effusions, biliary tumor cells and circulating tumor cells in whole blood. Subsequent analysis may identify therapeutic treatments based on a single cell analysis of activatable elements in cell signaling pathways. This analysis can be useful for diagnosis, prognosis, therapy selection and monitoring of solid tumor diseases.
Owner:NODALITY
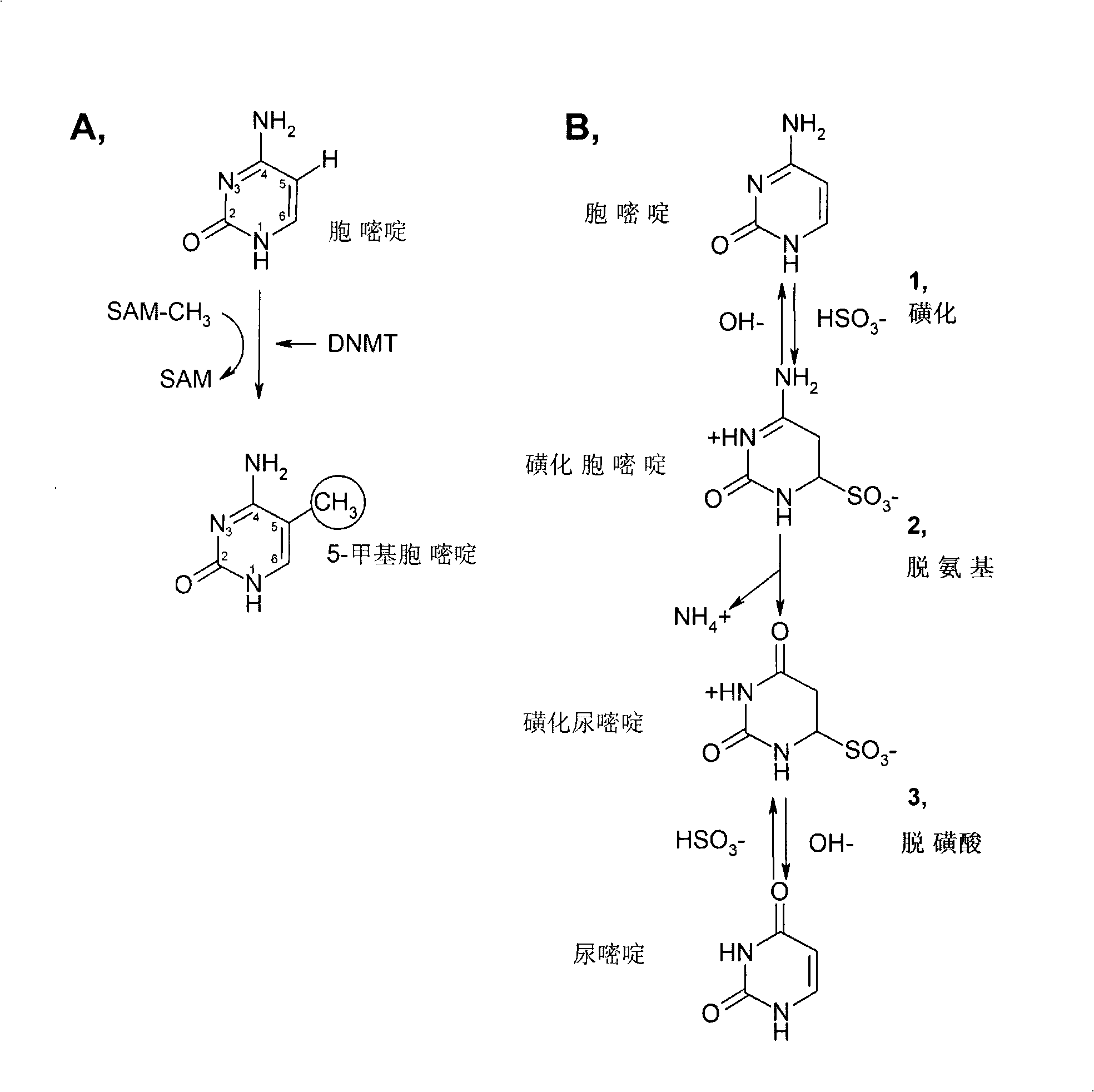
DNA methylation biomarker composition, detection method and kit
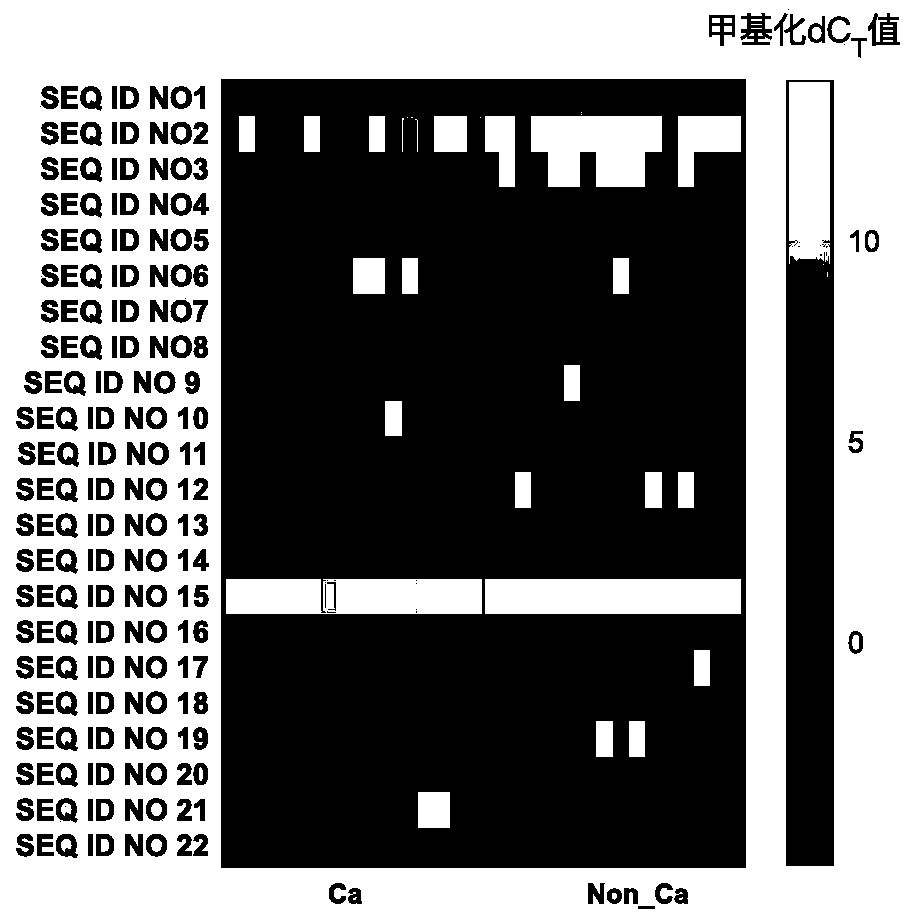
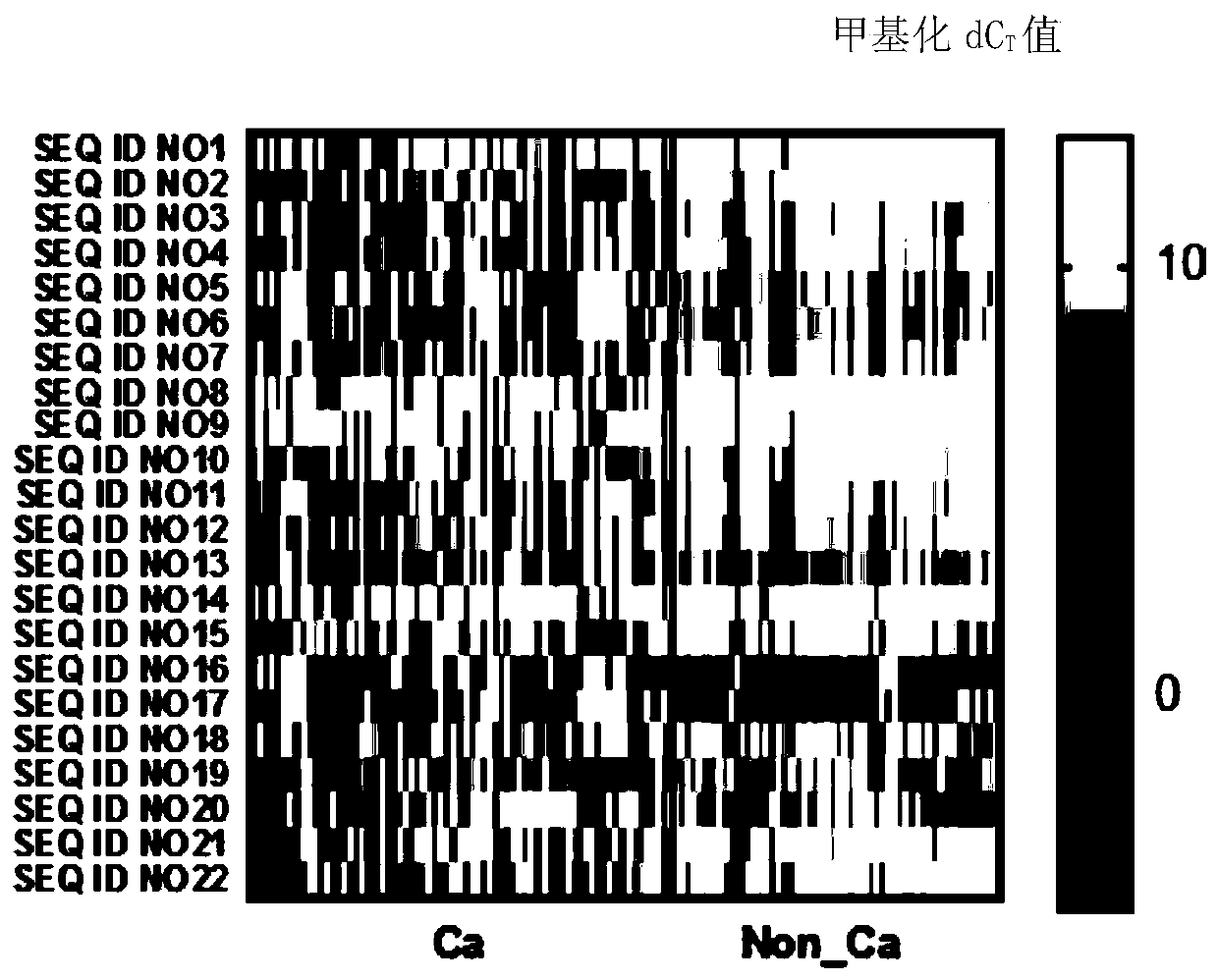
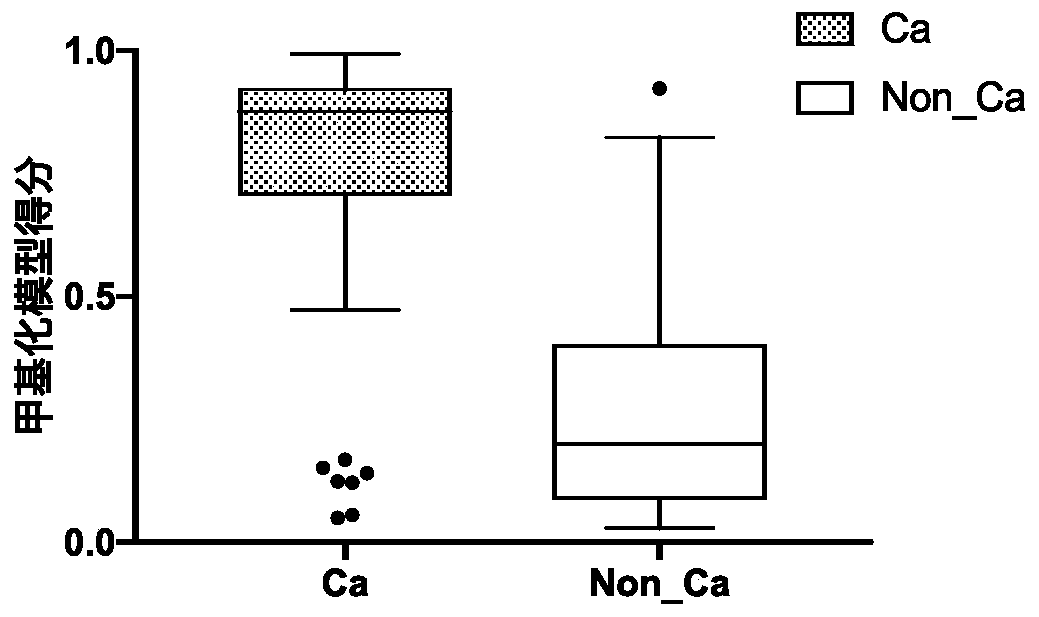
ActiveCN110872631AHigh sensitivityReact component optimizationMicrobiological testing/measurementDNA/RNA fragmentationDNA methylationMedicine
The invention relates to a DNA methylation biomarker composition for bladder cancer detection. The biomarker composition comprises any two or more of sequences shown in the formulas of SEQ ID NO.1 toSEQ ID NO.22, or any two or more of completely complementary sequences shown in the formulas of SEQ ID NO.1 to SEQ ID NO.22. The invention also relates to a detection kit for the methylation biomarkercomposition. The bladder cancer occurrence is discriminated and analyzed by using the composition in the co-methylation state of a plurality of specific methylation regions, the specific methylationcomposition has high sensitivity for discriminating the bladder cancer occurrence, and the detection method is simple, convenient and feasible. The primer pair composition of the kit overcomes the defect of false positive results caused by single methylation site detection mismatch in primer sequence design, and considers the interaction between multiple methylation biomarker primer and probe paircomposition.
Owner:ANCHORDX MEDICAL CO LTD
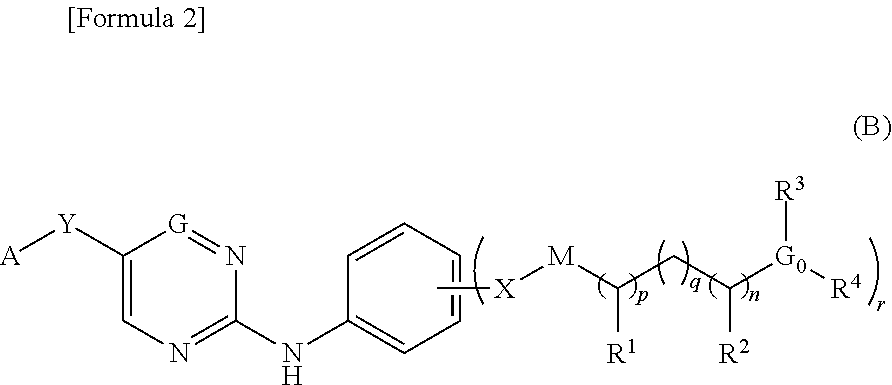
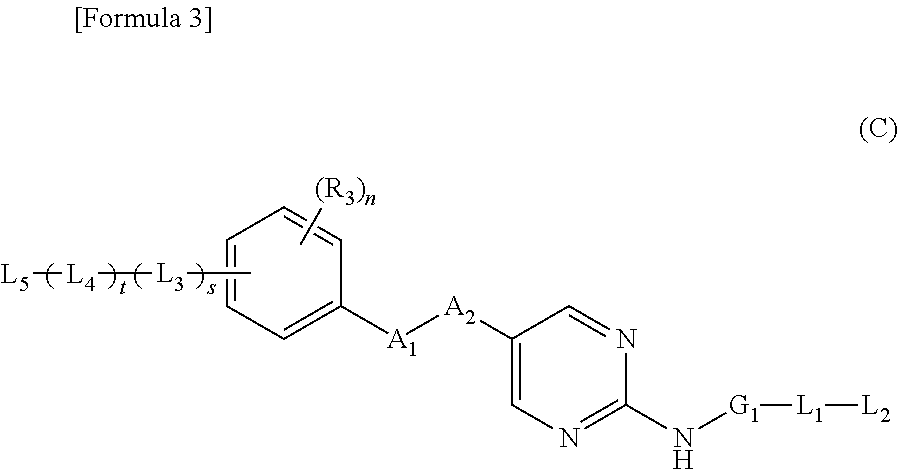
Nitrogen-containing aromatic heterocyclic compound
Provided is a compound useful as a prophylactic and / or therapeutic agent for bladder cancer.As a result of studies on compounds having FGFR inhibitory action, the present inventors have found that the nitrogen-containing aromatic heterocyclic compounds of the present invention have inhibitory action on FGFR1, FGFR2, and / or FGFR3, particularly, mutant FGFR3, and thus, the present invention has been accomplished. The nitrogen-containing aromatic heterocyclic compound of the present invention can be used as a therapeutic agent for various cancers related to FGFR1, FGFR2, and / or FGFR3, such as lung cancer and hormone therapy-resistant breast cancer, stomach cancer, triple negative breast cancer, endometrial cancer, bladder cancer, and glioblastoma, particularly as a prophylactic and / or therapeutic agent for mutant FGFR3-positive bladder cancer.
Owner:ASTELLAS PHARMA INC +1
36P6D5: secreted tumor antigen
InactiveUS7223542B2Improve the level ofOrganic active ingredientsPeptide/protein ingredientsProstate cancerTumor antigen
Owner:AGENSYS
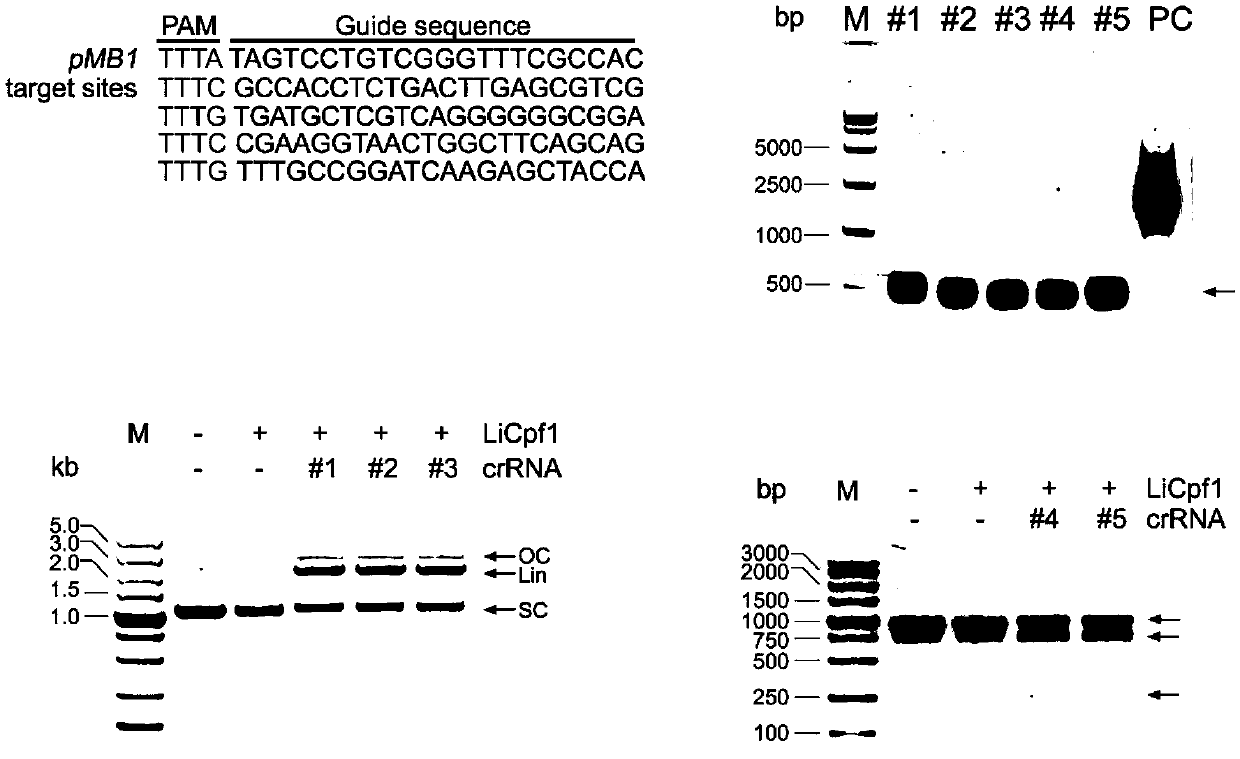
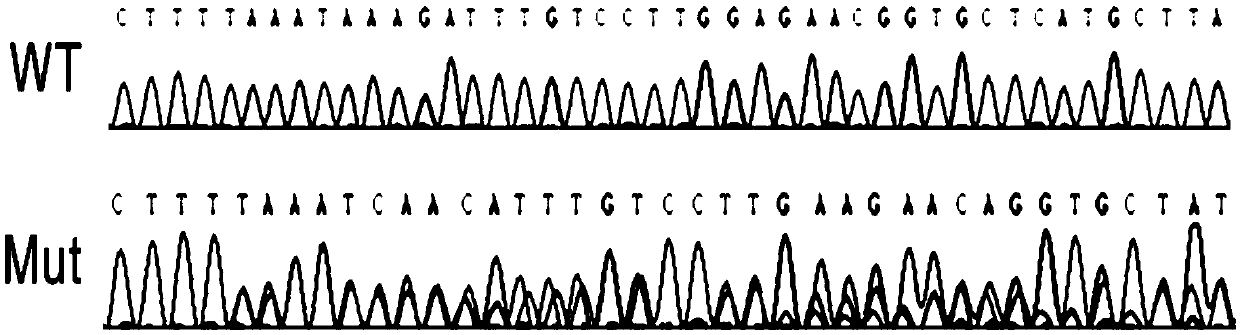
CRISPR/Cas12a gene editing system and application thereof
InactiveCN109666684AAchieve mutationWork lessHydrolasesStable introduction of DNATert geneEukaryotic cell
The invention relates to a CRISPR / Cas12a gene editing system and application thereof. The invention comprehensively utilizes biochemical, molecular biology, cell biology and other methods to screen and identify novel LiCas12a protein with endonuclease activity from a plurality of different bacteria. Based on LiCas12a, a CRISPR / LiCas12a gene editing system with high editing efficiency and high specificity is established in eukaryotic cells and prokaryotic cells separately, and genetic manipulation such as knockout, insertion and point mutation of genes is achieved; a molecular mechanism that the mutation of a TERT gene promoter promotes the cell proliferation of bladder cancer and liver cancer is disclosed. The genetic editing system based on CRISPR-LiCas12a provides a more accurate and efficient gene editing method for research fields such as disease pathogenesis and metabolic engineering transformation.
Owner:BEIJING UNIV OF CHEM TECH
Non-invasive detection method and kit for early screening bladder cancer by multi-gene combination
PendingCN108531594AHigh acceptanceImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationMultiple Tumor Suppressor-1Non invasive
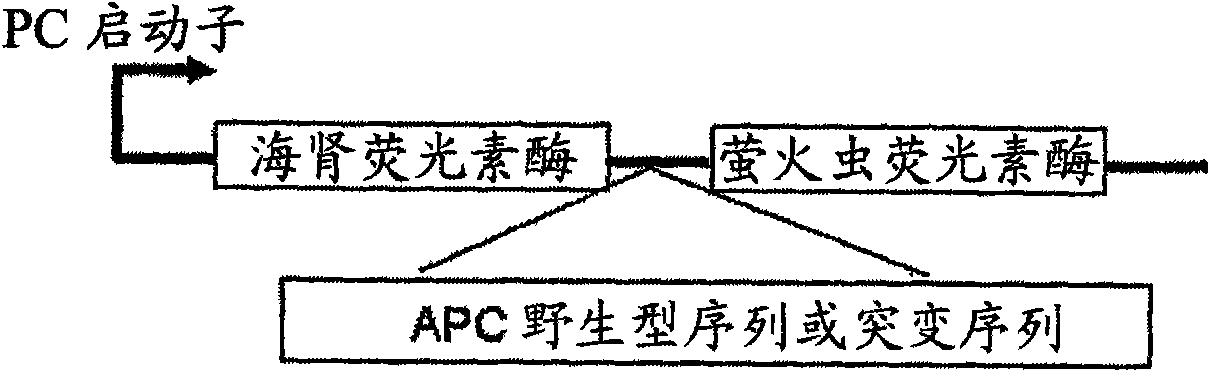
The invention discloses a non-invasive detection method and a kit for early screening a bladder cancer by multi-gene combination. The combined genes include APC, NID2 and p16, and specific methylationprimers are designed to detect three nucleotide sequences of target gene fragment methylation of the APC, the NID2 and the p16, so that early screening and auxiliary diagnosis of the bladder cancer are achieved. Methylation levels of three genetic markers of the APC, the NID2 and the p16 in urinary sediments are analyzed by a three-channel fluorescent quantitative PCR (polymerase chain reaction)method, and a positive or negative result of a sample is judged according to a reported CT (computed tomography) value. The non-invasive detection method can detect 90% of early bladder cancers, 64% of precancerous adenomas (larger than or equal to 1 centimeter) in preclinical study, specificity reaches 100%, invasiveness is completely omitted, the method only needs to collect urine of 50mL, and acceptability of throngs wearing no symptoms is high.
Owner:ANHUI DAJIAN MEDICAL TECH CO LTD
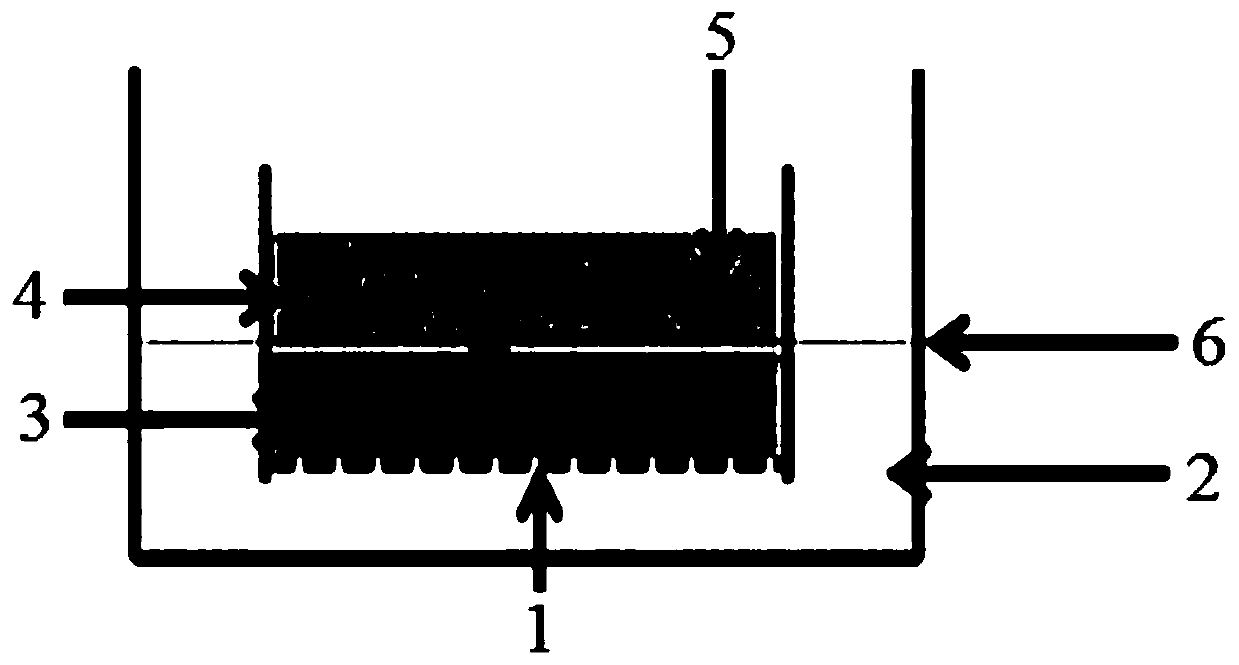
Method for culturing bladder cancer organs in vitro
ActiveCN111197030AImprove the success rate of cultivationAvoid conditions prone to hypoxiaCell dissociation methodsCell culture supports/coatingRat tailPharmaceutical drug
The invention discloses a method for culturing bladder cancer organs in vitro. The method comprises the following steps: manufacturing a gas-liquid interaction culture system, namely manufacturing a uniformly paved rat tail collagen supporting layer on the surface of a porous culture membrane in a Transwell upper chamber; re-suspending fresh in-vitro bladder cancer tissues by using a rat tail collagen solution, uniformly mixing, adding the obtained mixture onto the rat tail collagen supporting layer, and then putting the rat tail collagen supporting layer into an incubator at 37 DEG C to solidify so as to obtain a rat tail collagen layer containing the bladder cancer tissues; adding an organoid culture medium into a Transwell lower chamber, and enabling the liquid level of the culture medium to be lower than the rat tail collagen layer containing bladder cancer tissues; and carrying out passage and cryopreservation. The culture success rate of the bladder cancer organoid is remarkablyincreased, the bladder cancer organoid with reserved immune cells can be obtained through culture, operation is easy, the utilization rate of tumor tissue is high, and great significance and value areachieved for bladder cancer drug screening research.
Owner:上海嗣新医药科技有限公司
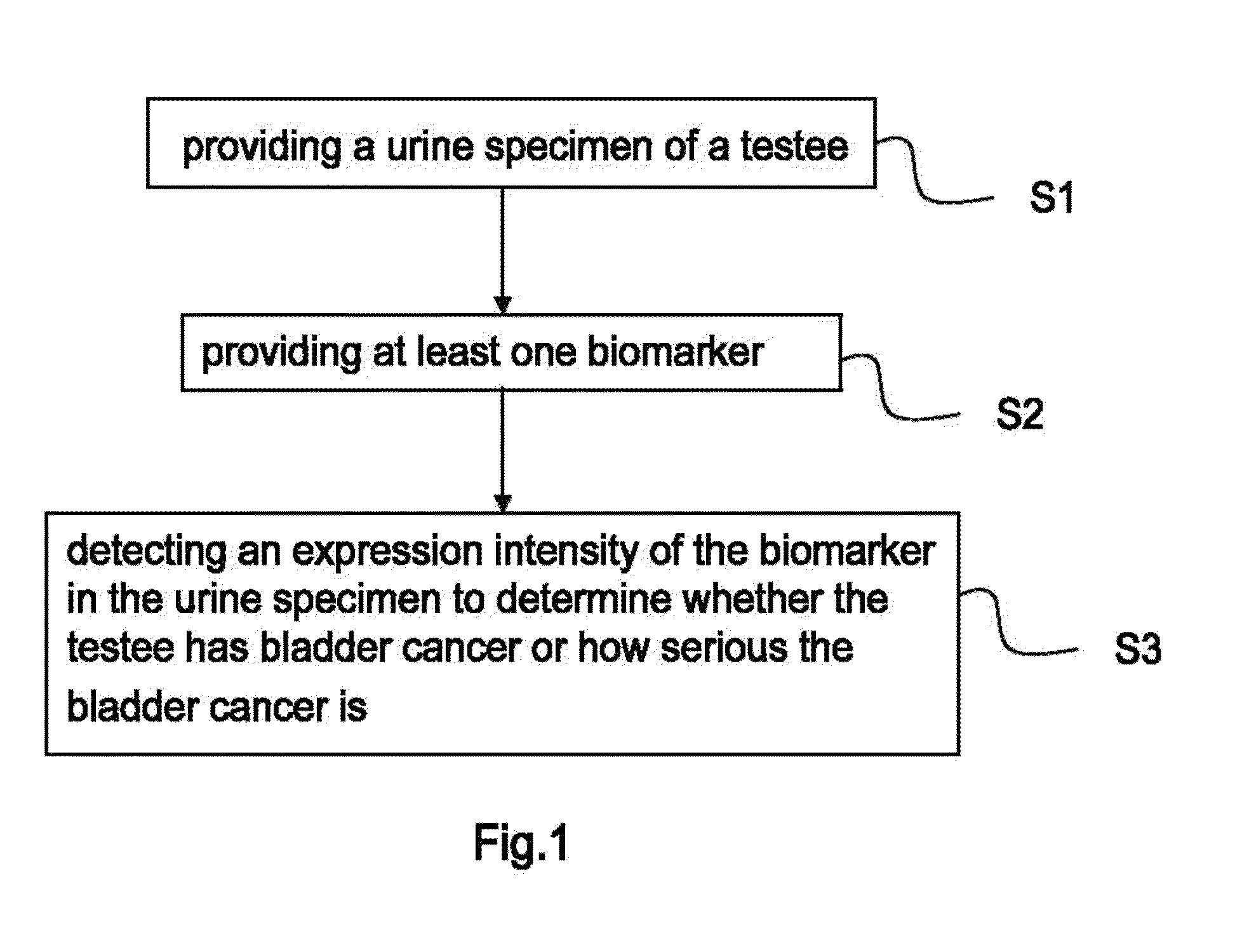
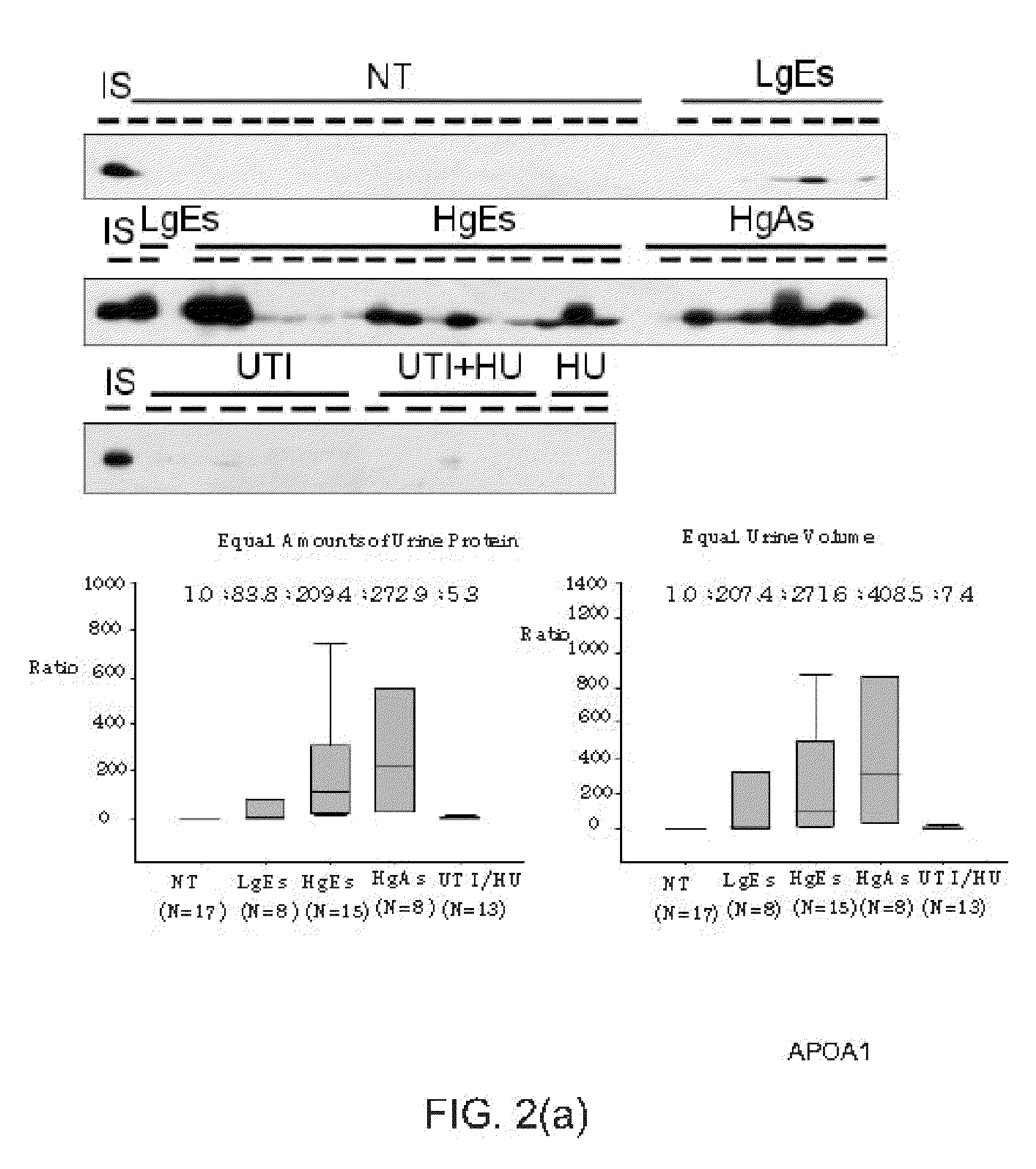
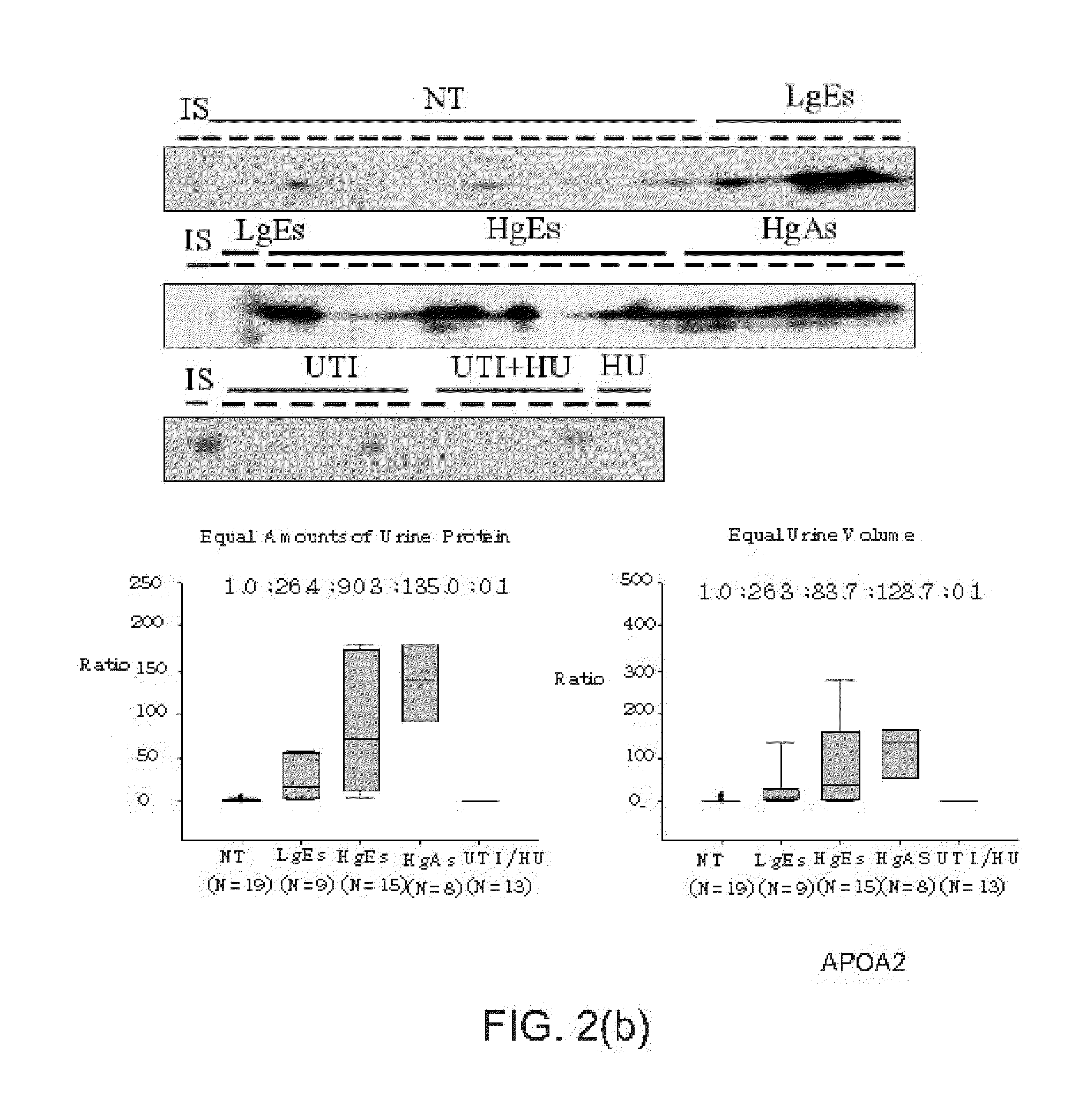
Bladder cancer biomarker and test method using the same
InactiveUS20110195478A1Diagnosing bladder cancerDetermining the aggressiveness of bladder cancerHydrolasesTransferasesHeparin cofactorTherapeutic effect
The present invention discloses a bladder cancer biomarker and a test method using the same. The biomarker contains at least one of the mentioned 69 compounds, such as apolipoprotein A1 (APOA1), apolipoprotein A2 (APOA2), peroxiredoxin 2 (PRDX2), heparin cofactor 2 precursor (HCII), and serum amyloid A-4 protein (SAA4), which exist in the urine specimen of a testee. The expression intensity of the biomarker can facilitate diagnosis of bladder cancer and evaluation of aggressiveness and malignancy of bladder cancer. Thereby, the physician can arrange an optimized treatment to achieve the best therapeutic effect.
Owner:CHANG GUNG UNIVERSITY
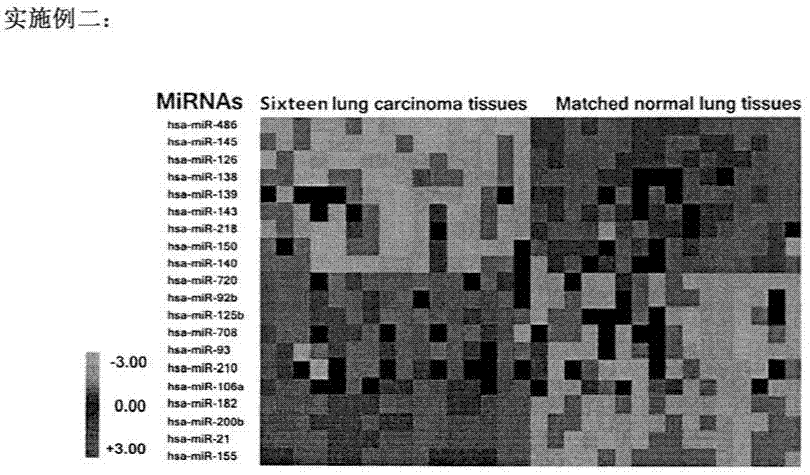
Detection of microRNAs in excreta as early diagnosis biomarker of lung cancer, colorectal cancer and bladder cancer
The invention discloses a method for detection of microRNAs in excreta (sputum, faeces and urine) as a biomarker for early screening and diagnosing lung cancer, colorectal cancer and bladder cancer. The method comprises: (1) screening abnormal expression microRNAs existed in tissues of the lung cancer, the colorectal cancer and the bladder cancer; (2) by using resection tumor samples from patients of the lung cancer, the colorectal cancer and the bladder cancer, verifying the screened microRNAs in the tumor tissues; and (3) detecting expressions of the microRNAs in samples of sputum, faeces and urine from the patients of the lung cancer, the colorectal cancer and the bladder cancer, analyzing correlation with expression in the tissues, and confirming the microRNAs for early screening and diagnosis. The method is substantially characterized in that the microRNAs in the excreta is taken as the biomarker for early screening and diagnosing the cancers, and the method helps to realize noninvasive screening for the cancer patients and possesses a wide application scope.
Owner:SHENZHEN PEOPLES HOSPITAL
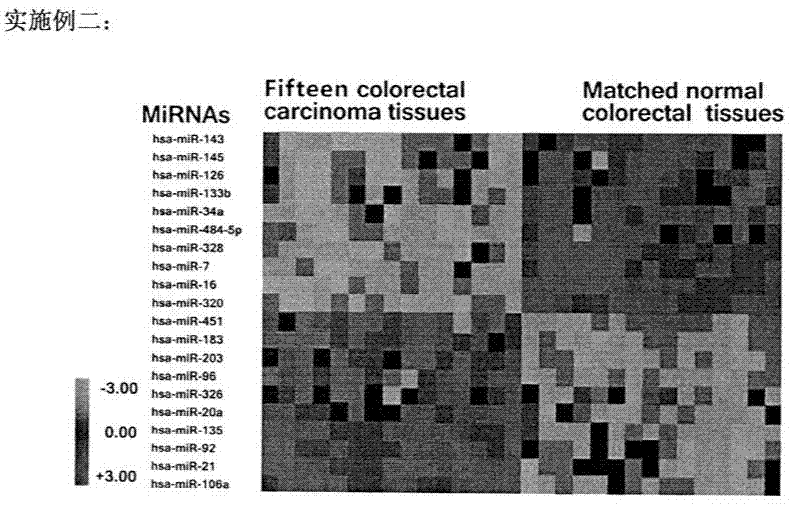
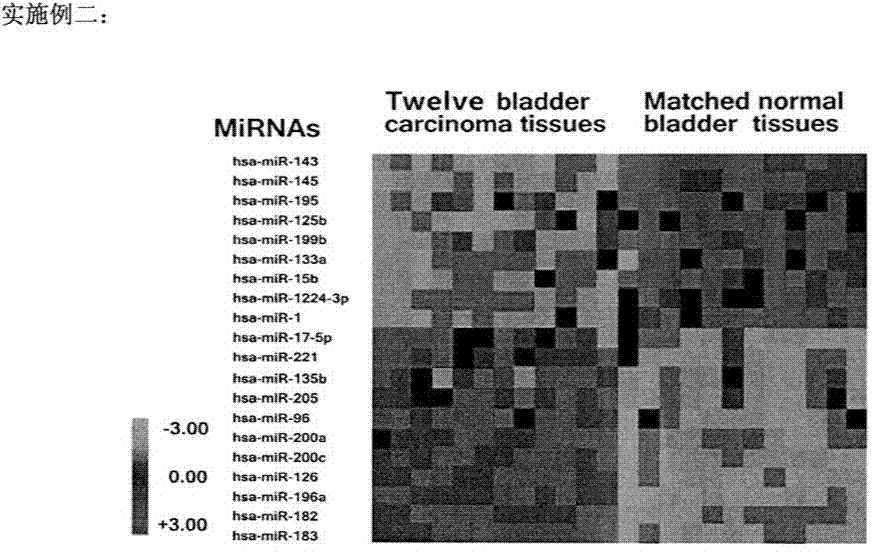
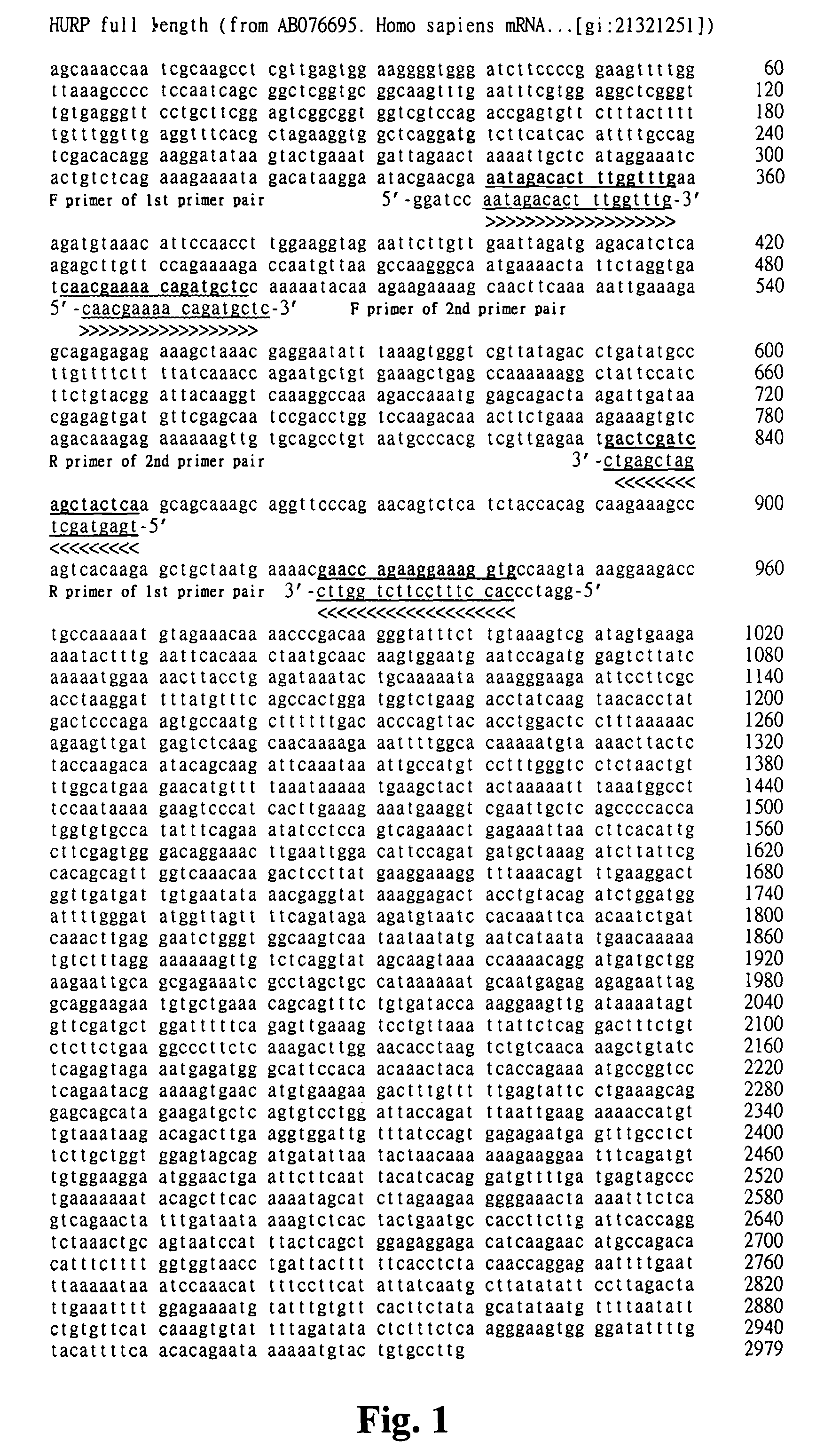
HURP gene as a molecular marker for bladder cancer
Hepatoma up-regulated protein (HURP) gene serves as a useful molecular marker in the detection, preliminary screening and monitoring of bladder cancer in a human subject. A method for the detection of bladder cancer, in particular transitional cell carcinoma (TCC) of the bladder, in a human subject is disclosed, in which the detected expression of the human HURP gene in a sample taken from a subject suspected to have bladder cancer is indicative of the presence of the bladder cancer. The present invention further provides a non-invasive method for the detection, preliminary screening or monitoring of urinary TCC in a suspected subject, in which a urine sample taken from the subject is analyzed to determine an expression of the human HURP gene, the presence of which is indicative of the presence of urinary TCC.
Owner:CHI MEI MEDICAL CENT
Methods for treating cancer
InactiveCN101568343ASuppression of premature termination mutationsOrganic active ingredientsAntineoplastic agentsMacrocyclic lactonePharmaceutical medicine
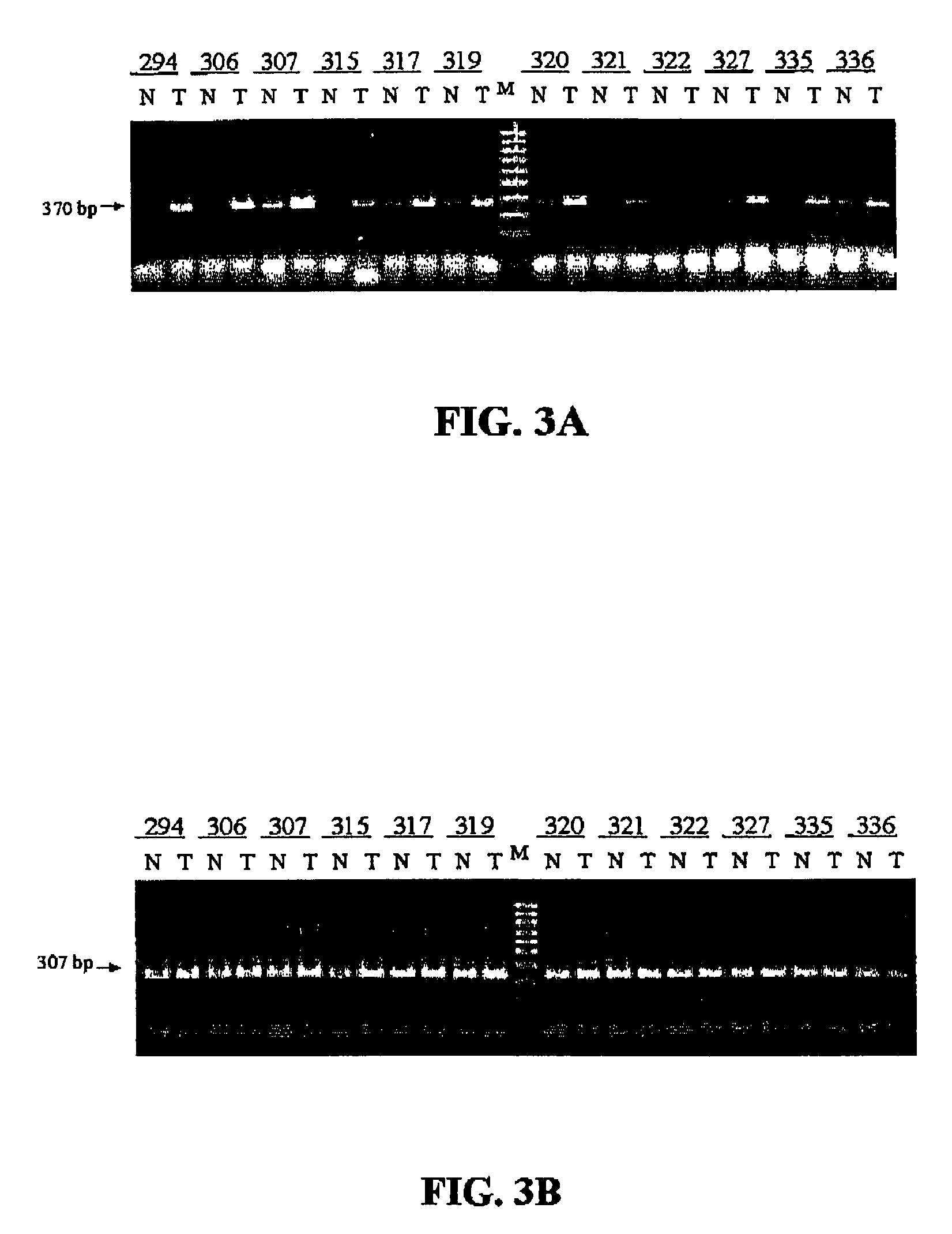
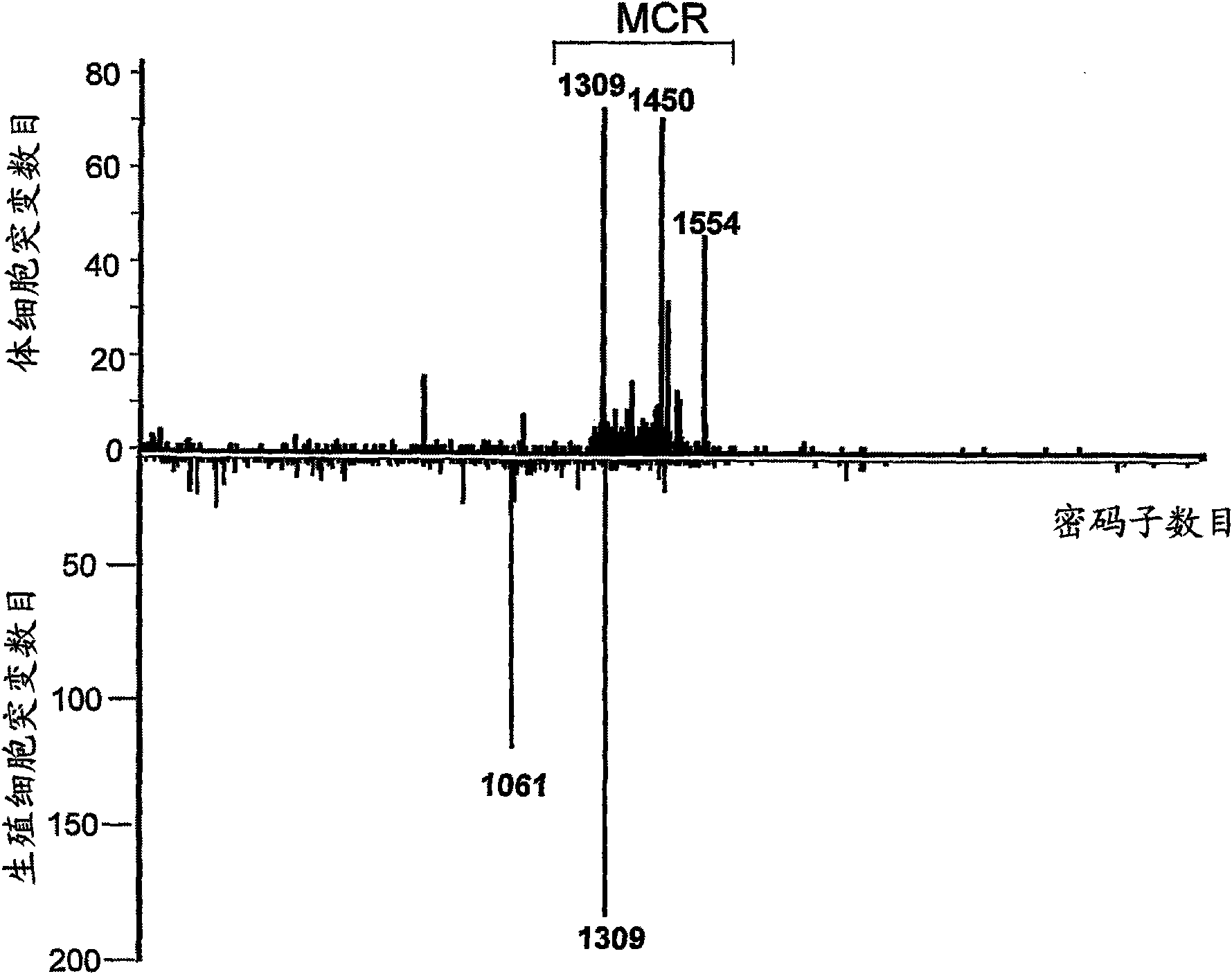
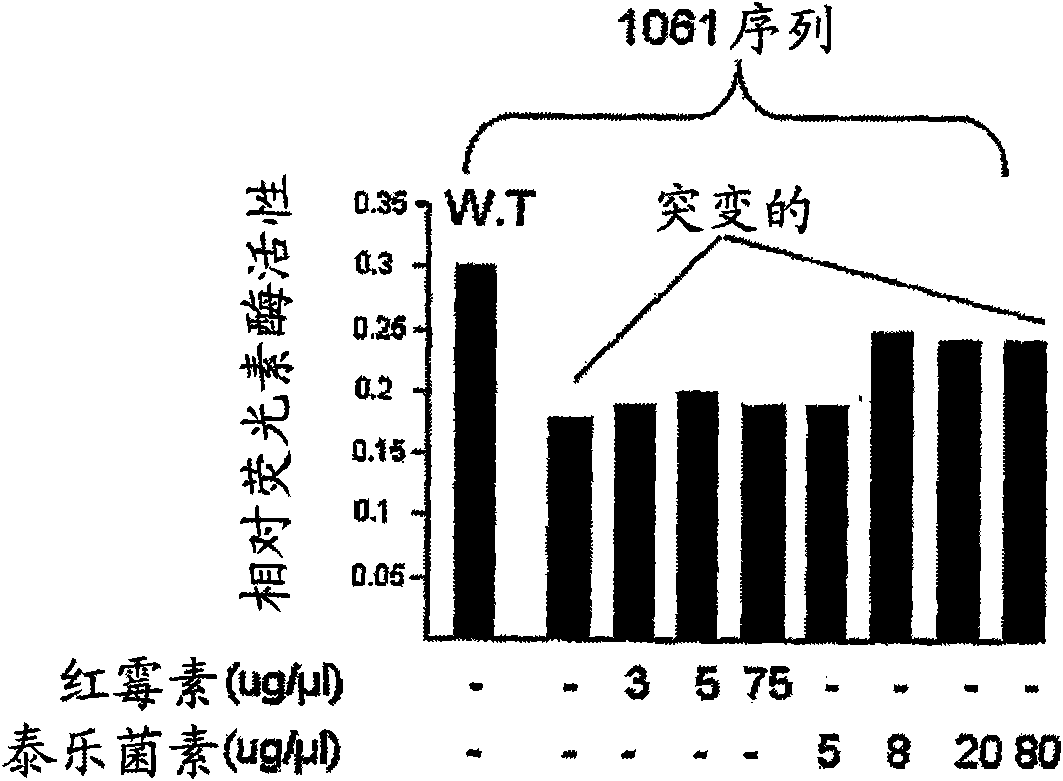
Use of a macrolide antibiotic for the manufacture of a medicament for the treatment or prevention of a cancer selected from colorectal cancer, Desmoid tumor, bladder cancer, gastric cancer, and breast cancer, the macrolide antibiotic being one or more of : tylosin, pharmaceutically acceptable salts thereof, and derivatives thereof; erythromycin, pharmaceutically acceptable salts thereof, and derivatives thereof; oleandomycin, pharmaceutically acceptable salts thereof, and derivatives thereof; and spiramycin, pharmaceutically acceptable salts thereof, and derivatives thereof. Also provided are pharmaceutical compositions and methods for the treatment or prevention of the above mentioned cancers and methods for treating or preventing, in a mammal, a cancer that expresses a mutated APC gene.
Owner:RAMOT AT TEL AVIV UNIV LTD
Method for reducing the likelihood of developing bladder or colorectal cancer in an individual human being
ActiveUS11026982B2Treatment complicationsAvoiding undesiredAntibacterial agentsOrganic active ingredientsPrevotellaMogibacterium diversum
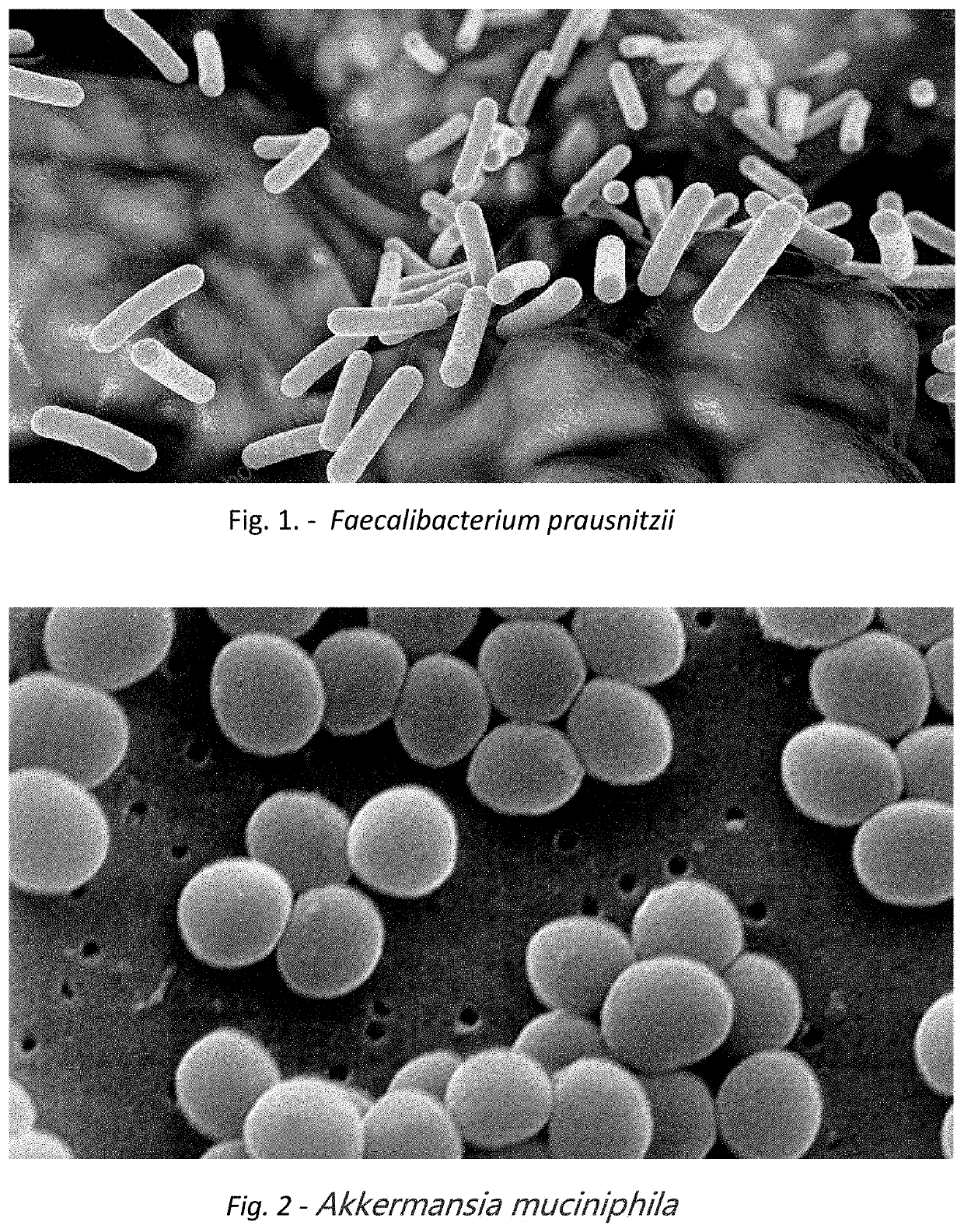
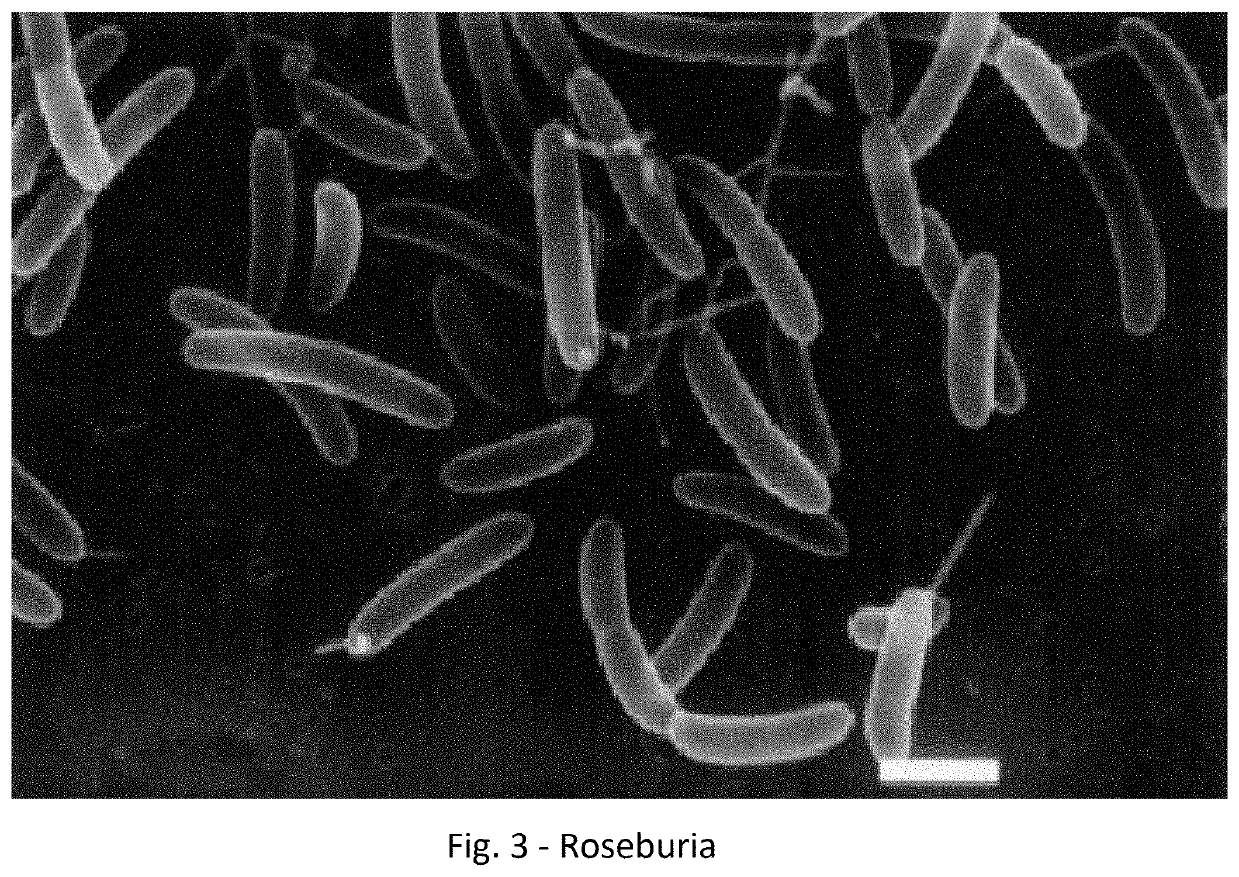
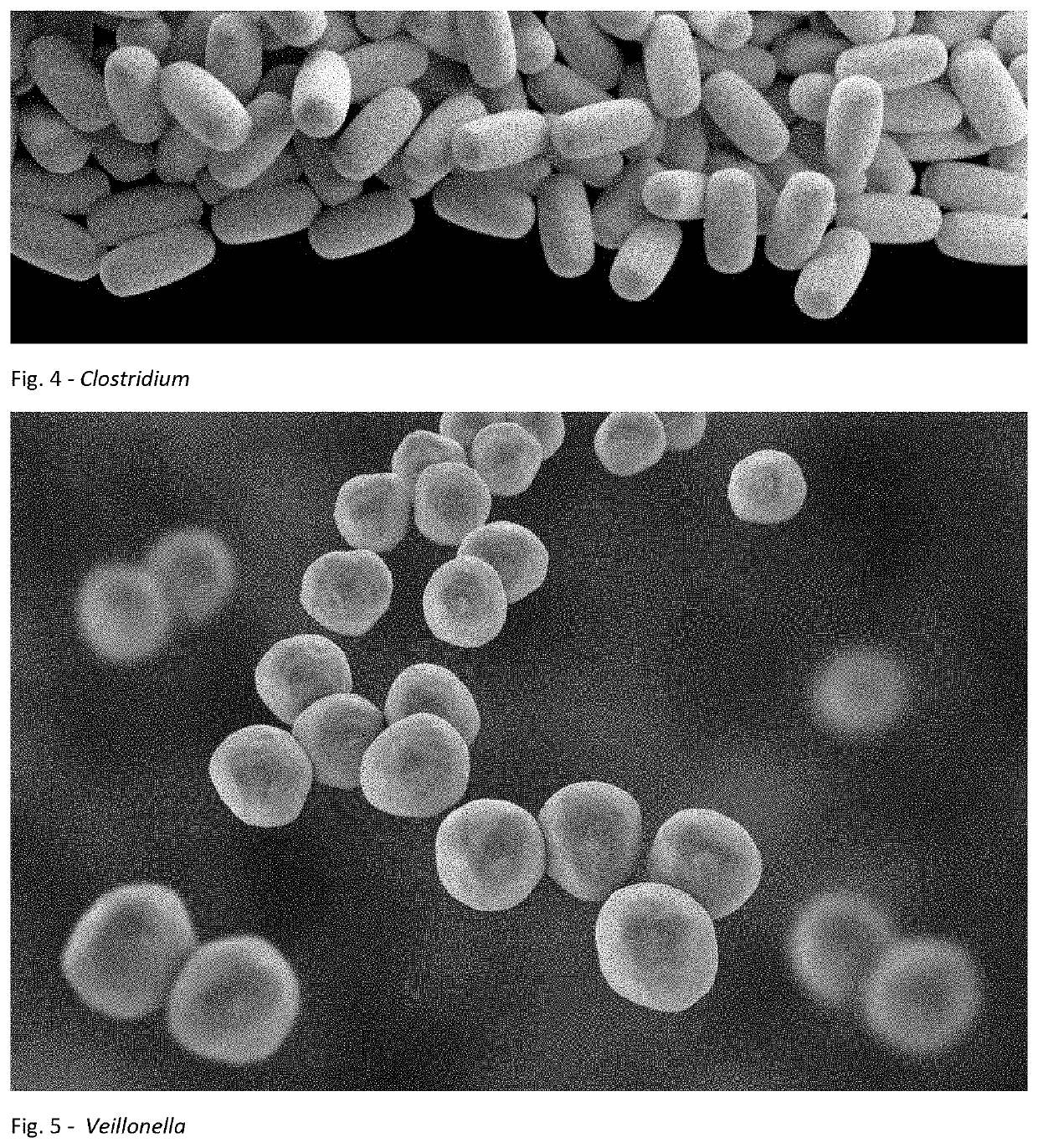
A method for treating an individual suffering from one of bladder cancer and colorectal cancer employs a CRISPR system to selectively kill or reduce the numbers of pathogenic bacteria within the individual and the individual is then administered an immune checkpoint inhibitor. In particular embodiments, the pathogenic bacteria is one of E. coli, Pseudomonas aeruginosa and Klebsiella bacteria, and the checkpoint inhibitor is selected from the group consisting of nivolumab, pembrolizumab, pidilizumab, AMP-224, AMP-514, STI-A1110, TSR-042, RG-7446, BMS-936559, MEDI-4736, MSB-0020718C, AUR-012 and STI-A1010. Further embodiments include enhancing the growth of a second bacteria in the individual, such bacteria including Akkermansia, Bacteroides, Bifidobacterium, Clostridium, Enterococcus, Fusobacterium, Lactobacillus, Propionibacterium, Ruminococcus, Veillonella, Prevotella, Escherichia and Streptococcus. Still other embodiments include increasing the levels of Roseburia and / or Faecalibacterium prausnitzii, in the individual's gut microbiome.
Owner:SEED HEALTH INC
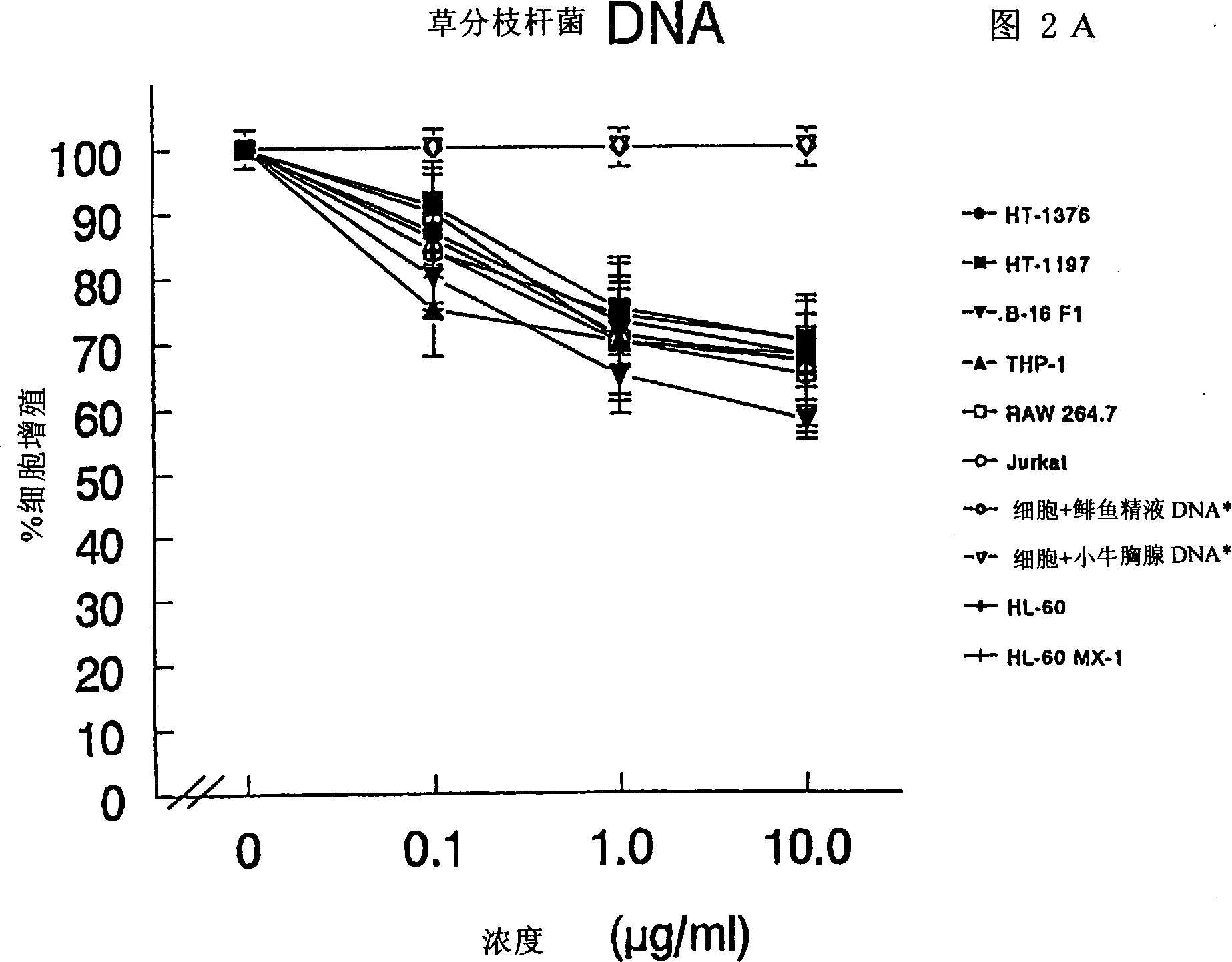
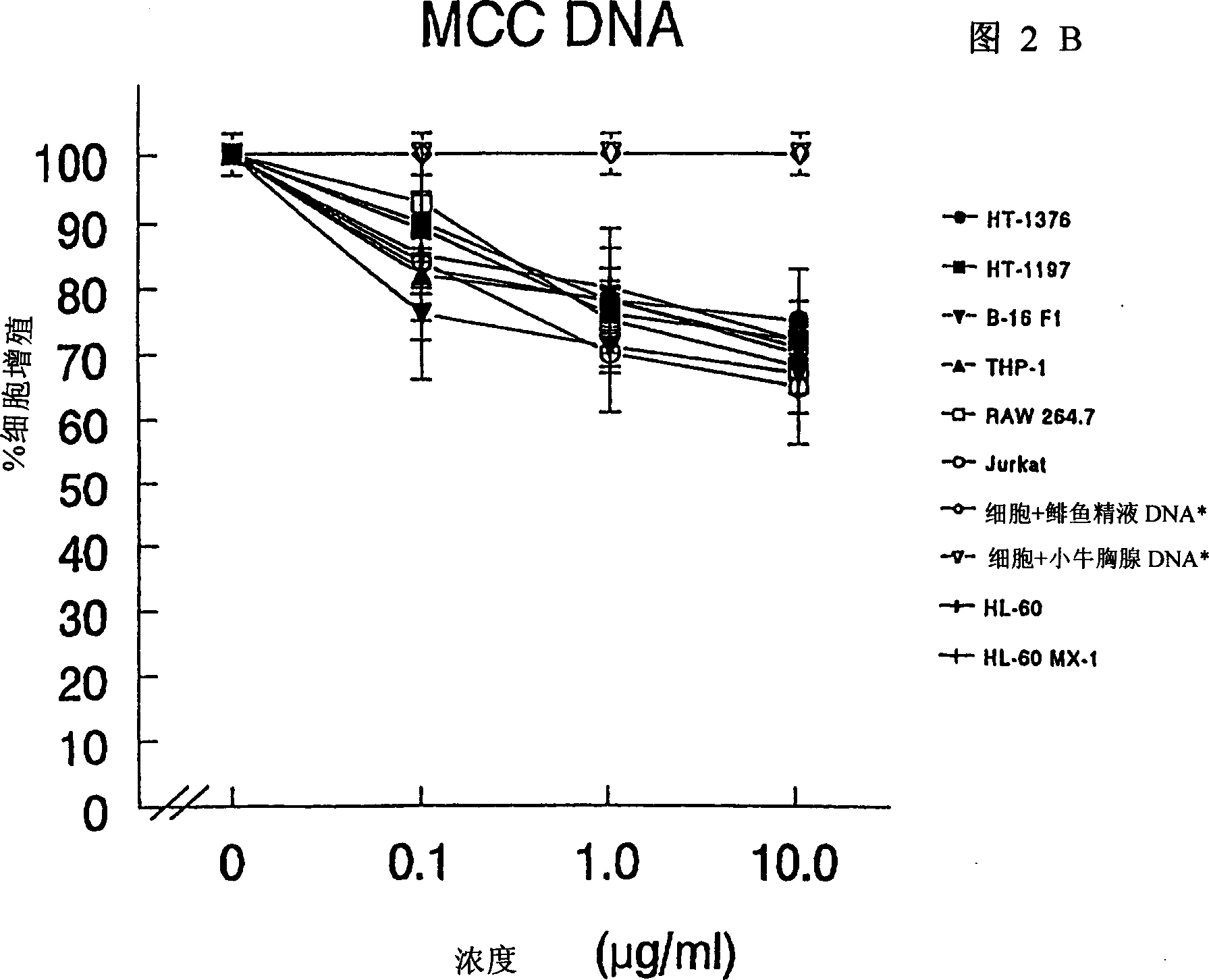
Composition and method for regulating cell proliferation and cell death
InactiveCN1290171ABacteria material medical ingredientsCarbohydrate active ingredientsMycobacterial cellCell wall
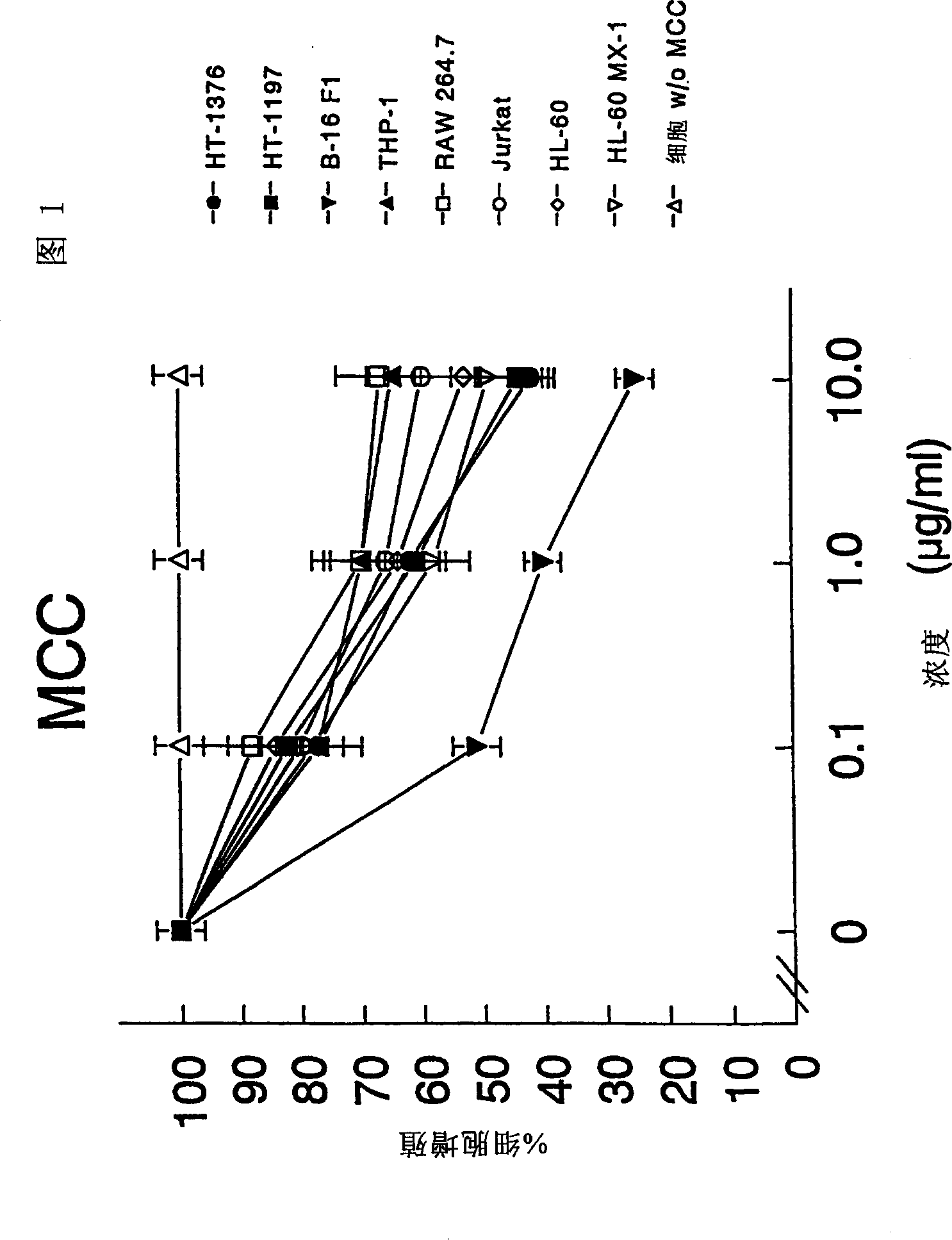
The present invention relates to a composition and method for treating cancer in the urinary bladder. More particularly, the present invention relates to a composition comprising a Mycobacterium phlei (M. phlei) deoxyribonucleic acid (M-DNA)-M. phlei cell wall complex (MCC), wherein the M-DNA is preserved and complexed on the M. phlei cell wall, and a pharmaceutically acceptable carrier. The MCC composition inhibits proliferation of and induces apoptosis in the cancer cells in the urinary bladder of the animal.
Owner:贝尔尼奇泌尿知识产权公司
Methods and compositions for delivery of pharmaceutical agents
InactiveUS7709457B2Good effectEnhanced transfectionBiocideOrganic active ingredientsCholesterolCyclodextrin
Methods and compositions for delivering pharmaceutical agents into cells, in particular urothelial cells of the bladder, are provided. In the methods and compositions of the invention, a solubilized cholesterol composition is used to facilitate delivery of pharmaceutical agents. Preferably, the cholesterol is solubilized by a cyclodextrin (e.g., methyl-β-cyclodextrin) and the pharmaceutical agent comprises a polynucleotide and either a cationic lipid, a cationic polymer or a dendrimer. Improved methods for transfecting polynucleotides into cells thus are also provided, using cationic lipids, cationic polymers or dendrimers and solubilized cholesterol, wherein the transfection efficiency is enhanced compared to use of cationic lipids, cationic polymers or dendrimers alone. Preferred methods of the invention involve transfecting polynucleotides into urothelial cells, preferably for therapeutic treatment of bladder cancer using, for example, a polynucleotide(s) encoding an interleukin(s), an interferon(s), a colony stimulating factor(s) and / or a tumor suppressor(s).
Owner:GENECURE PTE
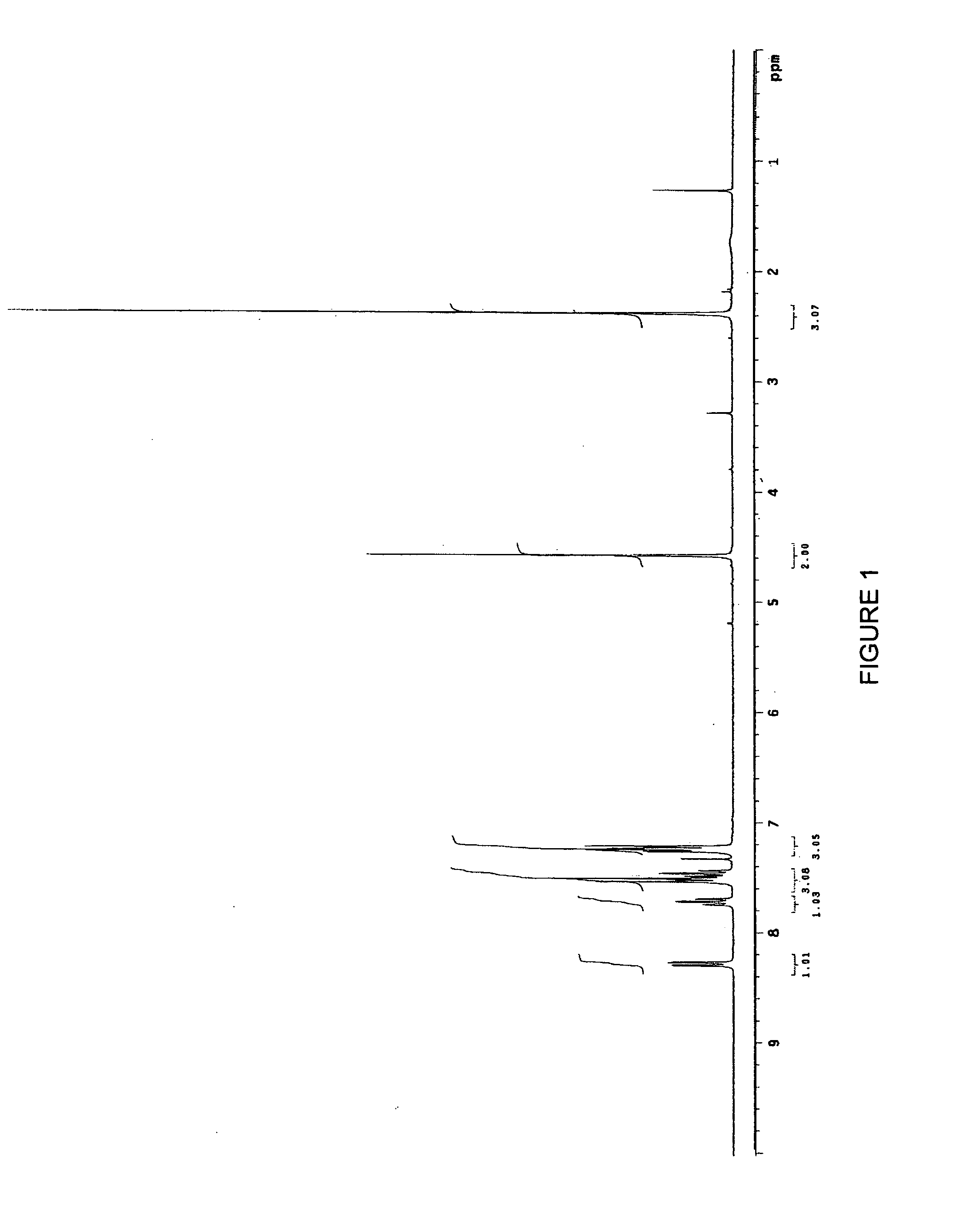
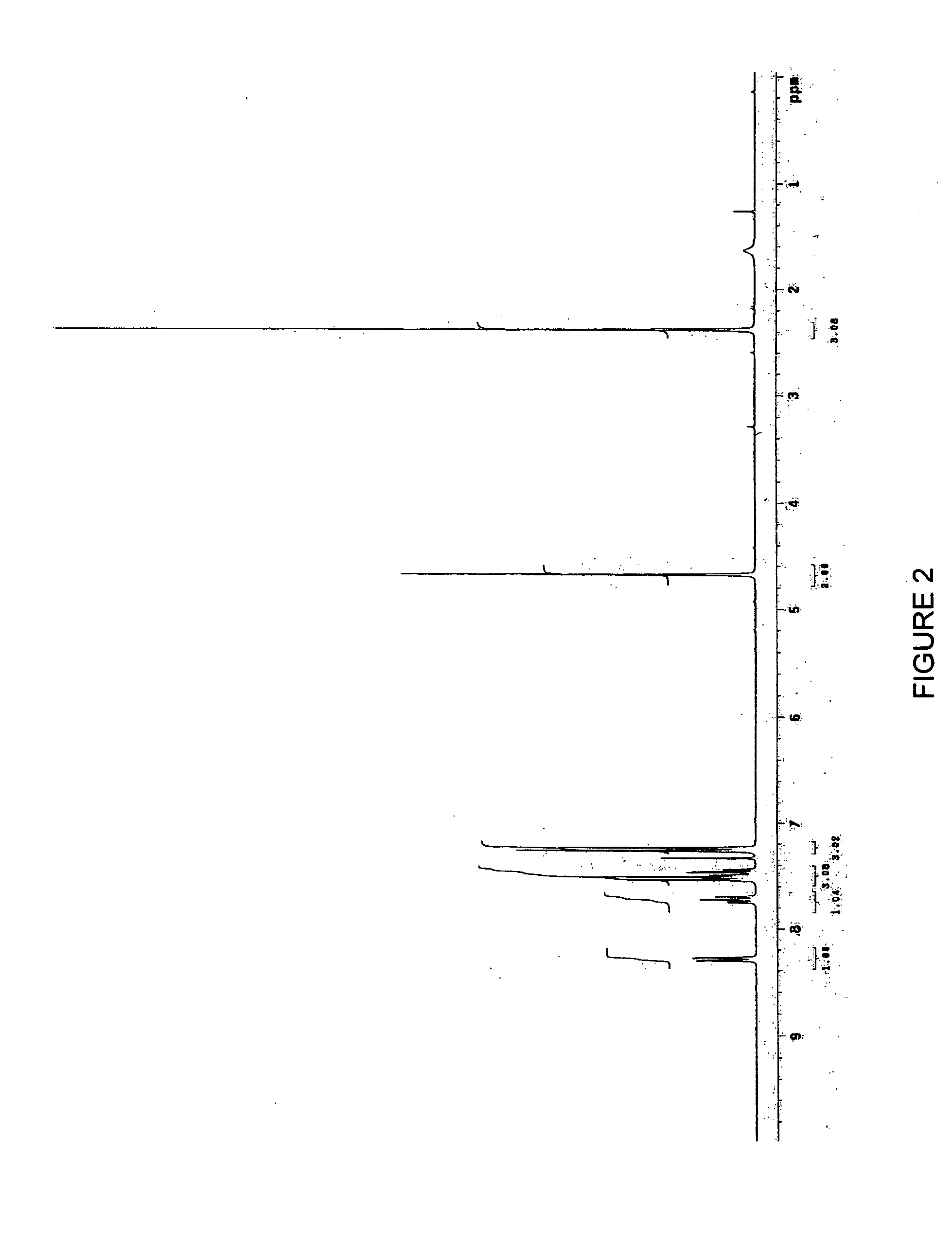
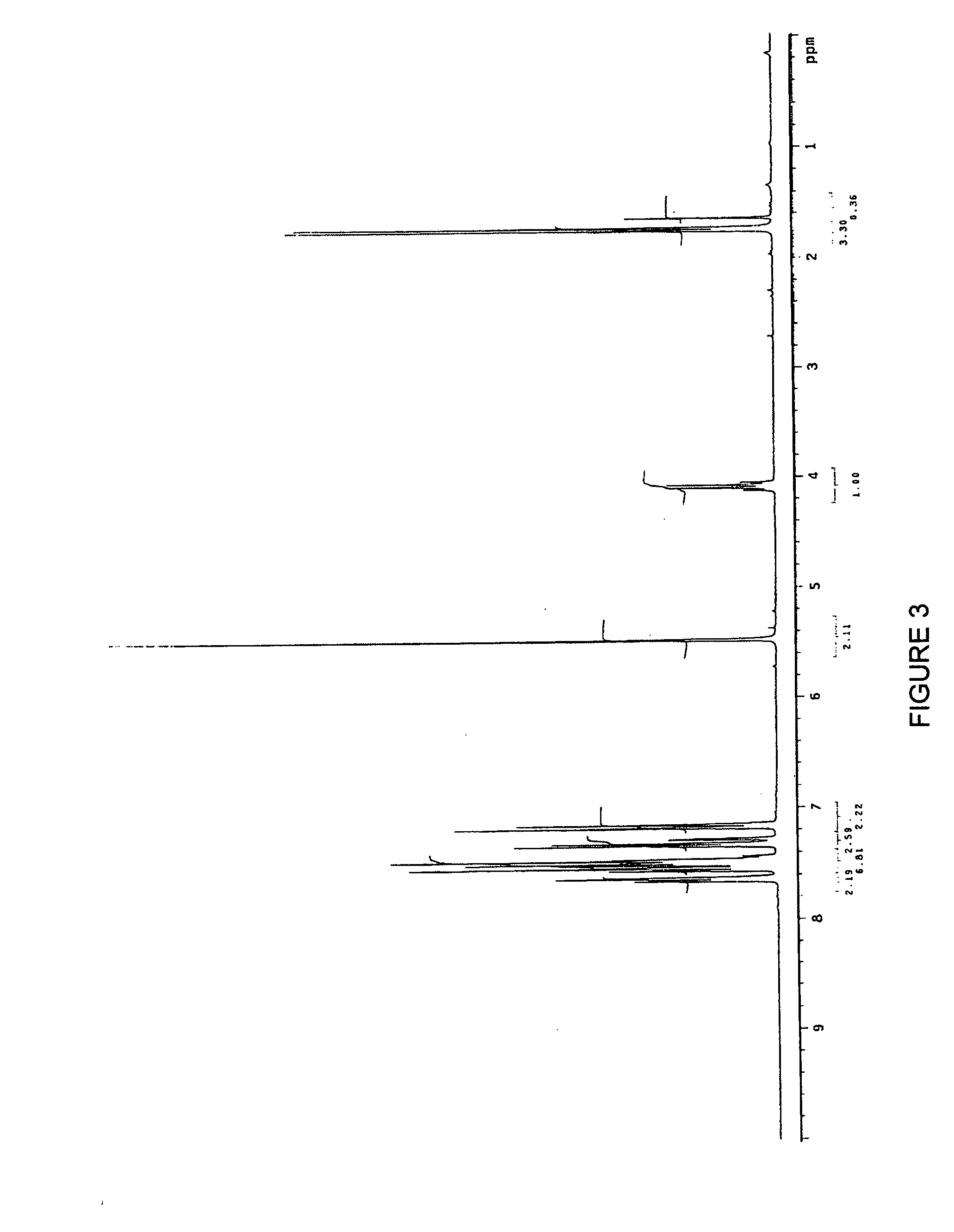
2-Indolyl Imidazo [4,5-d] Phenanthroline Derivatives and Their Use in the Treatment for Cancer
ActiveUS20100168417A1Prevent proliferationBiocideOrganic chemistryNon small lung cancerProstate cancer cell
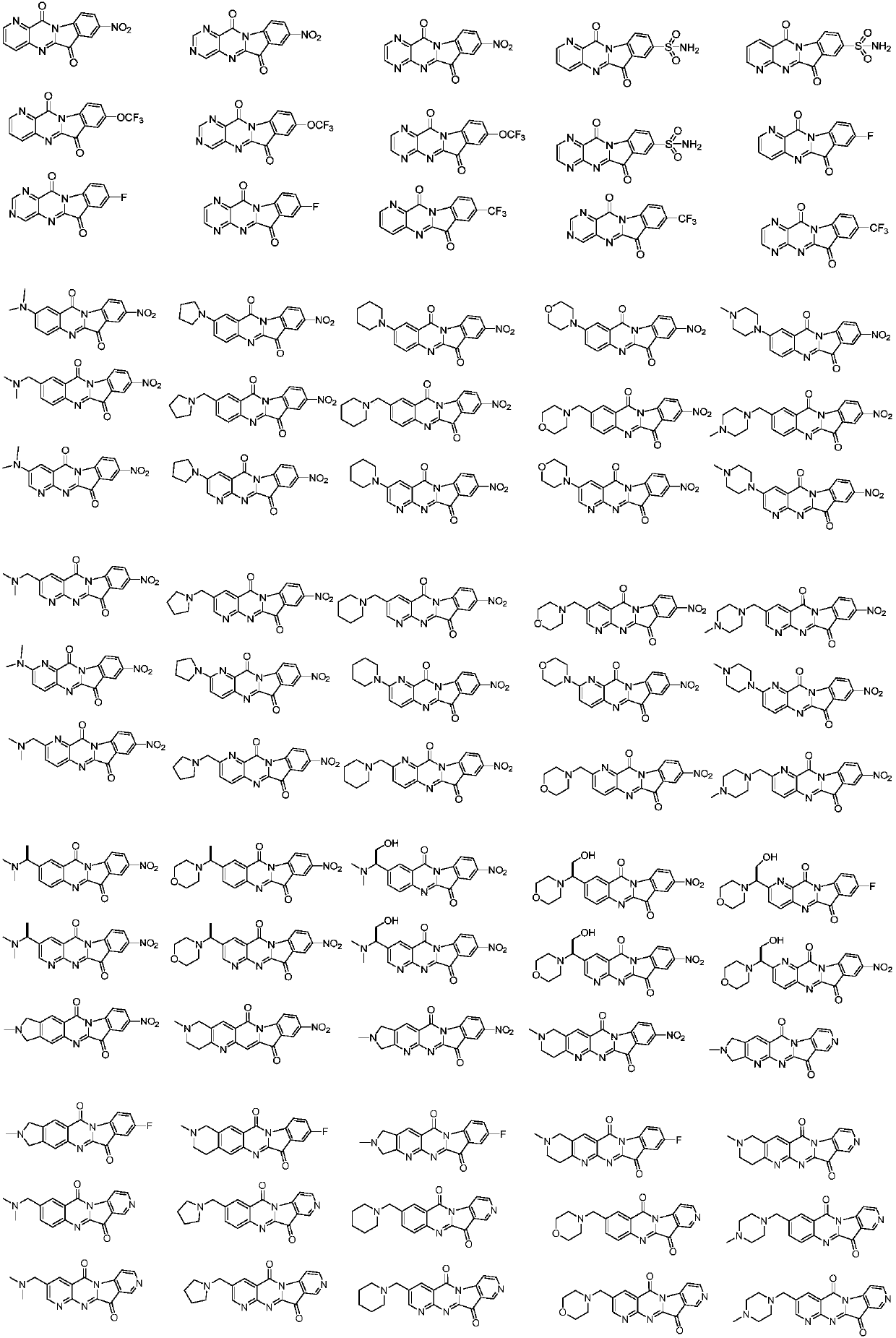
2-indolyl imidazo[4,5-d]phenanthroline compounds of Formula I that are capable of intracellular chelation of transition metals and of exerting antiproliferative effects in cancer cells, that are cytostatic and / or cytotoxic, are provided. Compounds of Formula I can also induce apoptosis in cancer cells and are thus capable of exerting a cytotoxic effect on cancer cells. The compounds of Formula I are also capable of selectively inhibiting the proliferation of one or more of prostate cancer cells, colon cancer cells, non-small lung cancer cells and leukemia cells. The compounds of Formula I are also capable of increasing the expression of the zinc-regulated tumour suppressor, KLF4 and thus are useful in inhibiting the proliferation of cancer cells in which KLF4 functions as a tumour-suppressor, including, but not limited to, bladder cancer, cancers of the gastrointestinal tract and various leukemias.
Owner:APTOSE BIOSCIENCES INC
Compounds and compositions for treating dysproliferative diseases, and methods of use thereof
Compounds are disclosed with activity towards killing dysproliferative cells in vitro and treating cancer in vivo. Cancers such as cancer of the colon, pancreas, prostate, lung, breast, urinary bladder, skin and liver are exemplary. Compounds, pharmaceutical compositions and methods of use are described.
Owner:CHESTERFORD ENTERPRISES
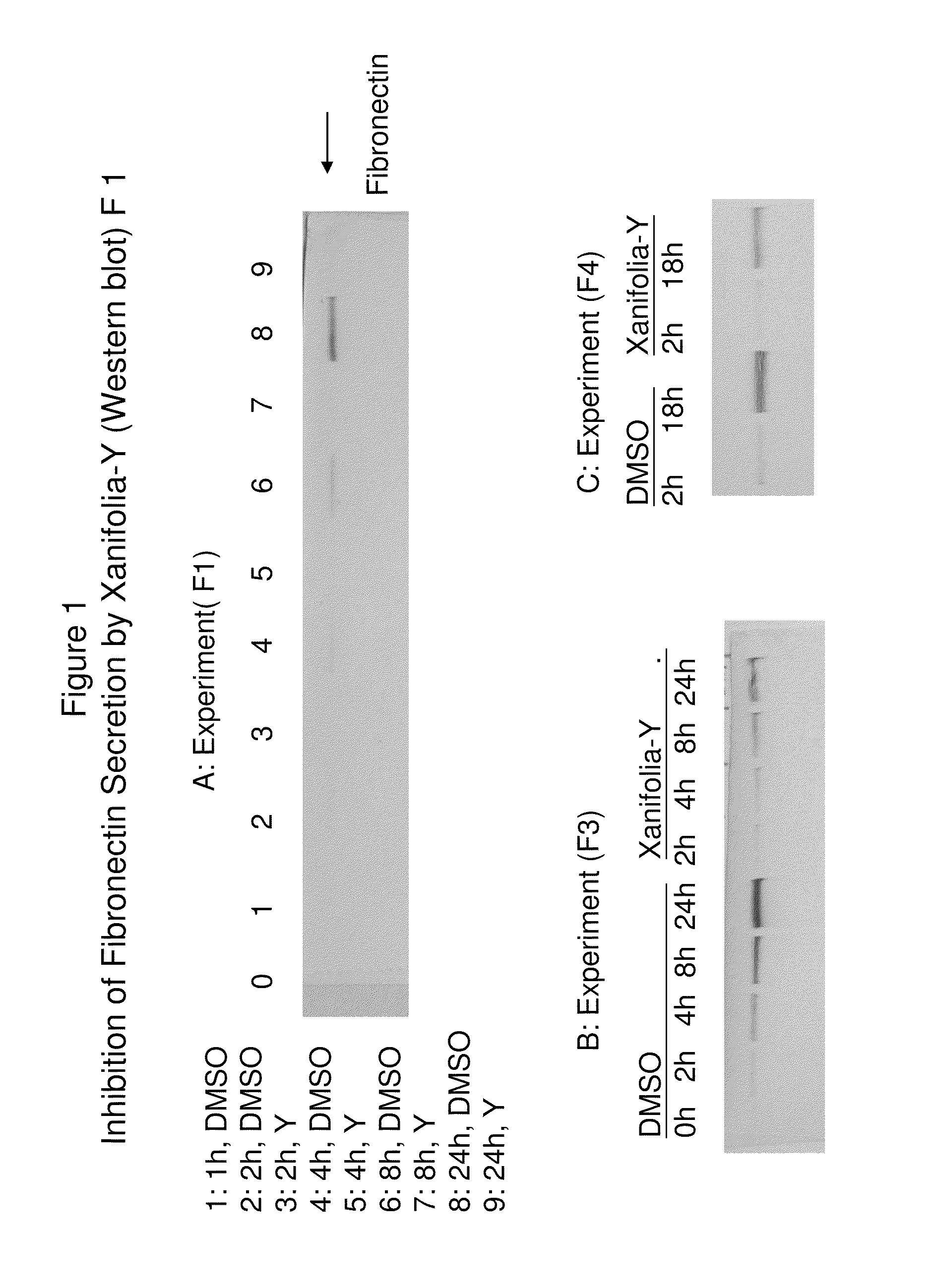
Blocking the migration or metastasis of cancer cells by affecting adhesion proteins and the uses of new compounds thereof
This invention provides methods, processes, compounds and compositions for modulating the gene expression and modulating the secretion, expression, or synthesis of adhesion proteins or their receptors to cure disease, wherein the modulating comprises positive and negative regulating; wherein comprises inhibiting cancer growth, wherein the adhesion proteins or receptors comprise fibronectin, integrins family, Myosin, vitronectin, collagen, laminin, Glycosylation cell surface proteins, polyglycans, cadherin, heparin, tenascin, CD 54, CAM, elastin and FAK; wherein the methods, processes, compounds and compositions are also for anti-angiogenesis; wherein the cancers comprise breast cancer, leukocyte cancer, liver cancer, ovarian cancer, bladder cancer, prostate cancer, skin cancer, bone cancer, brain cancer, leukemia cancer, lung cancer, colon cancer, CNS cancer, melanoma cancer, renal cancer or cervix cancer.
Owner:PACIFIC ARROW
VHZ for diagnosis and treatment of cancers
We provide VHZ for use in a method of treatment, prophylaxis or alleviation of a cancer in an individual selected from the group consisting of: colon cancer, lung cancer, squamous cell carcinoma including lip, larynx, vulva, cervix and penis cancer, pancreatic cancer, brain cancer, oesophageal cancer, stomach cancer, bladder cancer, kidney cancer, skin cancer, ovary cancer, prostate cancer and testicular cancer. We provide an anti-VHZ agent for the treatment, prophylaxis or alleviation of such a cancer. The anti-VHZ agent may comprise SEQ ID NO:4 or SEQ ID NO: 5, or both.
Owner:AGENCY FOR SCI TECH & RES
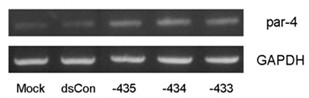
Application of target up-regulation PAR-4 gene small ribonucleic acid (RNA) in preparing bladder cancer resisting drugs
InactiveCN102641508AEasy to operateLow costGenetic material ingredientsAntineoplastic agentsApoptosisTumor cells
The invention provides application of target up-regulation PAR-4 gene small ribonucleic acid (RNA) in preparing bladder cancer resisting drugs. The nucleotide sequence of the target up-regulation PAR-4 gene small RNA is composed of six positive sense strands and negative sense strands of 21 nucleotides. The 3' end of each strand is provided with two nucleotides in suspension mode (generally dTdT, and the 19 nucleotides in the middle are paired.). The target up-regulation PAR-4 gene small RNA comprises sa RNA of different sequences. By means of expression of target up-regulation PAR-4 genes in the bladder cancer cells, the small RNA can restrain tumor cell activity, induces cell apoptosis, and further can be applied to preparation of bladder cancer resisting drugs. The application of target up-regulation PAR-4 gene small RNA in preparing the bladder cancer resisting drugs is simple in operation of preparing the small RNA, low in cost, small in using amount, capable of achieving good activation effect when the transfection concentration is 50nM and accurate in activation action. The mRNA and protein level of the target genes are both improved. In addition, the sa RNA can induce genes to express without damaging completeness of gene groups, thereby being safe to use.
Owner:ZHEJIANG UNIV
Application of cedilanid in preparation of anti-liver cancer and anti-bladder cancer medicament
InactiveCN102416019AReduce doseMinor topical application toxicityOrganic active ingredientsPharmaceutical delivery mechanismOncologyCardiac glycoside
The invention discloses an application of cedilanid in the preparation of anti-liver cancer and anti-bladder cancer medicaments; cedilanid is prepared into an injection, and is used for liver cancer and bladder cancer resistance; the concentration IC50 of the injection is 8*10-4 mmol / L. According to the invention, in an in-vitro experiment, the IC50 of cedilanid for liver cancer and bladder cancer cells is 8*10-4 mmol / L, and the effect is better than other cardiac glycoside medicaments; when compared with a common medicament of 5-fluorouracil with an IC50 of 1*10-1 mmol / L, cedilanid has a potency stronger by 100 times; in an antitumor experiment in animal body, cedilanid has a tumor inhibition rate of 34.26-48.43%; if application methods are proper, cedilanid is an ideal anticancer medicament.
Owner:GUILIN MEDICAL UNIVERSITY
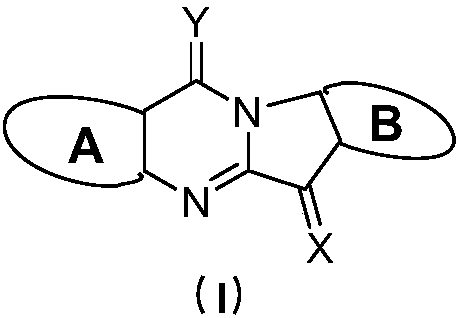
Tryptanthrin derivatives and application thereof
InactiveCN109956942AStrong inhibitory activityGood treatment effectOrganic active ingredientsSenses disorderDiseaseHead and neck tumors
The invention discloses compounds represented by a general formula (I) shown in the description, wherein in the formula (I), A and B are respectively a six-membered cyclic compound containing D1, D2,D3 and D4 and a six-membered cyclic compound containing D5, D6, D7 and D8. The invention also disclose an indoleamine-2,3-dioxygenase and / or tryptophan-2,3-dioxygenase inhibitor containing the above compounds and the application of the compounds in preparation of medicaments for treating cancers. The compounds provided by the invention can effectively inhibit cell proliferation, and have good therapeutic effects on various diseases such as the cancers, significant therapeutic effects on breast cancer, cervical cancer, colon cancer, lung cancer, stomach cancer, rectal cancer, pancreatic cancer,brain cancer, skin cancer, oral cancer, prostate cancer, bone cancer, kidney cancer, ovarian cancer, bladder cancer, liver cancer, fallopian tube tumors, ovarian tumors, peritoneal tumors, stage IV melanoma, glioma, neuroblastoma, hepatocellular carcinoma, mastoid renal tumors, head and neck tumors, leukemia, lymphoma, myeloma and non-small cell lung cancers, and very broad application prospects.
Owner:SHANGHAI SHENGYUE PHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






![2-Indolyl Imidazo [4,5-d] Phenanthroline Derivatives and Their Use in the Treatment for Cancer 2-Indolyl Imidazo [4,5-d] Phenanthroline Derivatives and Their Use in the Treatment for Cancer](https://images-eureka.patsnap.com/patent_img/5b62d250-8752-49d5-a36e-f88e437ae6c6/US20100168417A1-20100701-D00000.png)
![2-Indolyl Imidazo [4,5-d] Phenanthroline Derivatives and Their Use in the Treatment for Cancer 2-Indolyl Imidazo [4,5-d] Phenanthroline Derivatives and Their Use in the Treatment for Cancer](https://images-eureka.patsnap.com/patent_img/5b62d250-8752-49d5-a36e-f88e437ae6c6/US20100168417A1-20100701-D00001.png)
![2-Indolyl Imidazo [4,5-d] Phenanthroline Derivatives and Their Use in the Treatment for Cancer 2-Indolyl Imidazo [4,5-d] Phenanthroline Derivatives and Their Use in the Treatment for Cancer](https://images-eureka.patsnap.com/patent_img/5b62d250-8752-49d5-a36e-f88e437ae6c6/US20100168417A1-20100701-D00002.png)