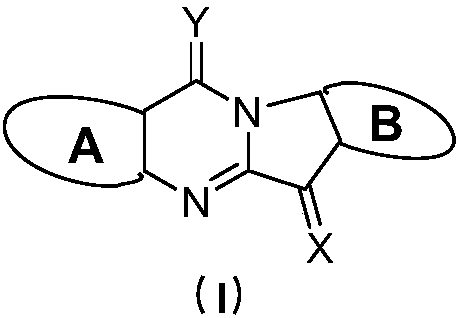
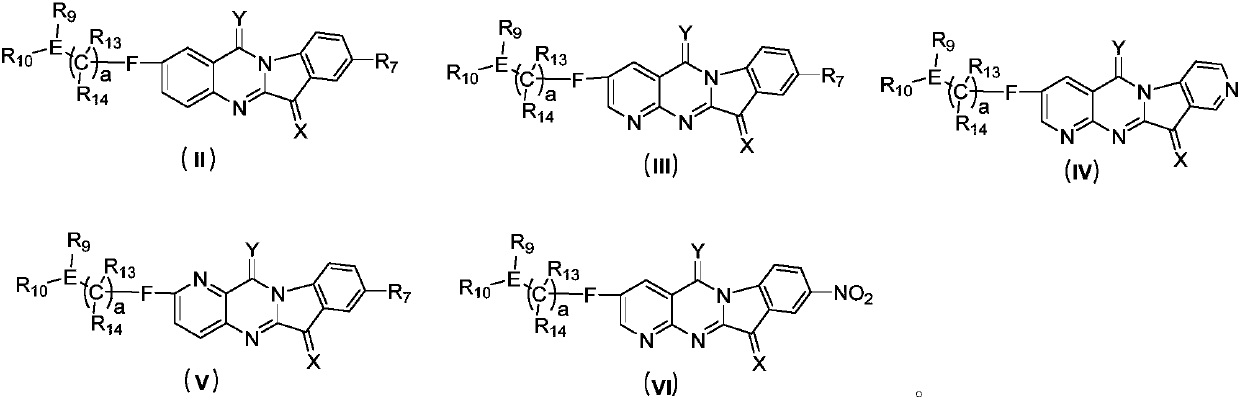
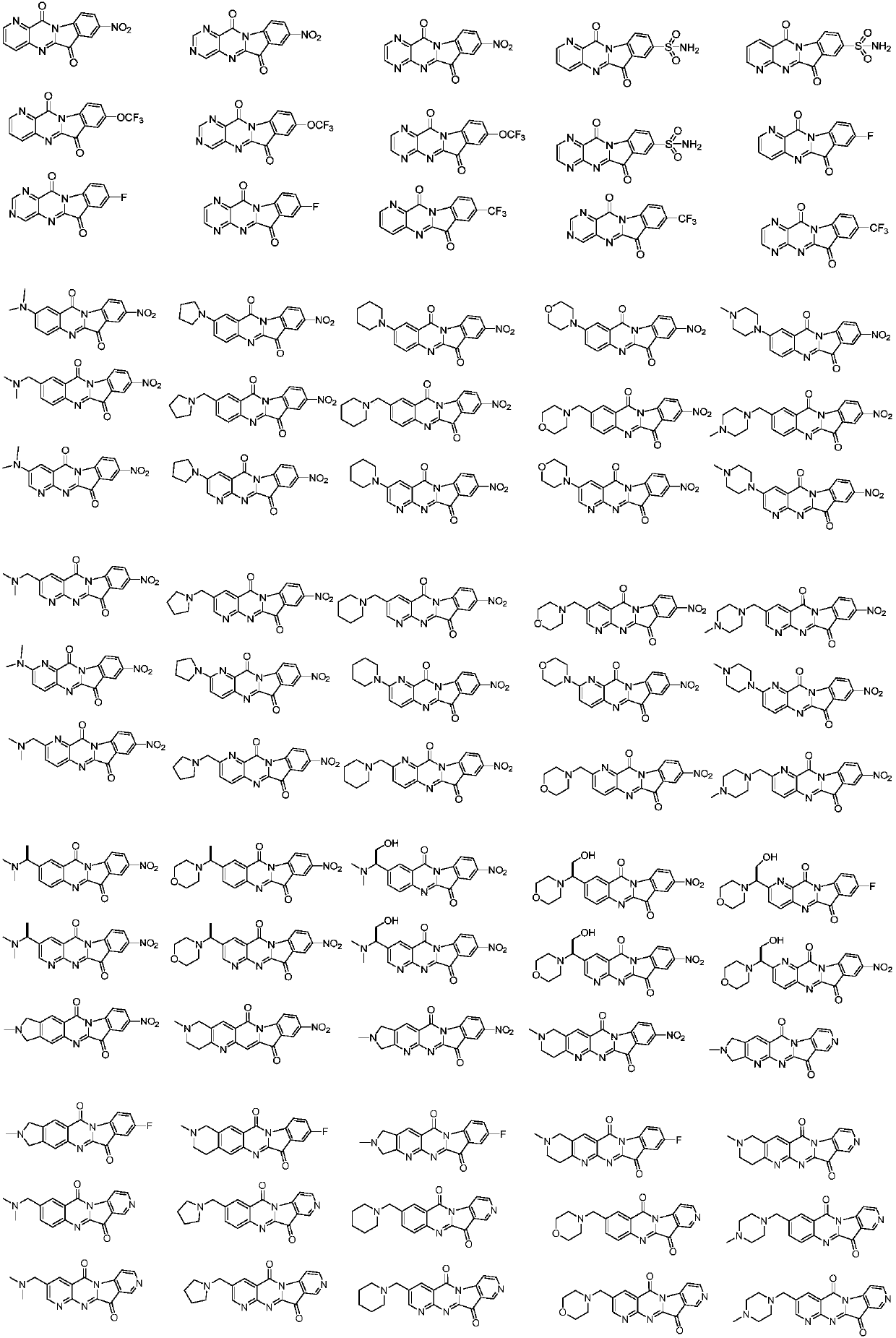
Tryptanthrin derivatives and application thereof
A compound and mixture technology, applied in the field of medicine, can solve problems such as T-cell activation signal transduction blockage, achieve good therapeutic effect, broad application prospects, and inhibit cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Synthesis of 8-nitropyrido[3',2':4,5]pyrimido[1,2-a]indole-6,12-dione
[0041]
[0042] 5-Nitroindole-2,3-dione (192mg, 1mmol) and 1,8-diazabicycloundec-7-ene (304mg, 2mmol) were dissolved in N,N-dimethyl Formamide (1 mL), stirred at room temperature for ten minutes. Dissolve 3-aminopyridine-2-carboxylic acid (138mg, 1mmol), N-methylmorpholine (202mg, 2mmol), O-benzotriazole-tetramethyluronium hexafluorophosphate (380mg, 1mmol) in In N,N-dimethylformamide (2 mL), the reacted solution of isatin and DBU was added dropwise to the solution, and stirred at room temperature for 20 hours. The solvent was evaporated to dryness and separated by column chromatography (dichloromethane:methanol=80:1) to obtain an orange-yellow solid (400mg, 67%) which was 8-nitropyrido[3',2':4,5]pyrimido [1,2-a]indole-6,12-dione (Compound 3).
[0043] LC-MS: 295[M+H] +
Embodiment 2
[0044] Example 2 Synthesis of 8-nitropyrimido[5',4':4,5]pyrimido[1,2-a]indole-6,12-dione
[0045]
[0046] 5-Nitroindole-2,3-dione (192mg, 1mmol) and 1,8-diazabicycloundec-7-ene (304mg, 2mmol) were dissolved in N,N-dimethyl Formamide (1 mL), stirred at room temperature for ten minutes. Dissolve 5-aminopyrimidine-4-carboxylic acid (138mg, 1mmol), N-methylmorpholine (202mg, 2mmol), O-benzotriazole-tetramethyluronium hexafluorophosphate (380mg, 1mmol) in In N,N-dimethylformamide (2 mL), the reacted solution of isatin and DBU was added dropwise to the solution, and stirred at room temperature for 20 hours. The solvent was evaporated to dryness and separated by column chromatography (dichloromethane:methanol=80:1) to obtain an orange-yellow solid (403mg, 67%) which was 8-nitropyrimido[5',4':4,5]pyrimido [1,2-a]indole-6,12-dione (Compound 5).
[0047] LC-MS: 296[M+H] +
Embodiment 3
[0048] Example 3 Synthesis of 8-nitroindole [2,1-b] pteridine-6,12-dione
[0049]
[0050] 5-Nitroindole-2,3-dione (192mg, 1mmol) and 1,8-diazabicycloundec-7-ene (304mg, 2mmol) were dissolved in N,N-dimethyl Formamide (1 mL), stirred at room temperature for ten minutes. Dissolve 3-aminopyrazine-2-carboxylic acid (139mg, 1mmol), N-methylmorpholine (202mg, 2mmol), O-benzotriazole-tetramethyluronium hexafluorophosphate (380mg, 1mmol) In N,N-dimethylformamide (2 mL), the reacted solution of isatin and DBU was added dropwise to the solution, and stirred at room temperature for 20 hours. The solvent was evaporated to dryness and separated by column chromatography (dichloromethane:methanol=80:1) to obtain an orange-yellow solid (412mg, 69%) which was 8-nitroindole[2,1-b]pteridine-6,12 - diketones (compound 7).
[0051] LC-MS: 296[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com