Two-step <99m>Tc-ECD marking process with high radiochemical purity and <99m>Tc-ECD solution
A 99mtc-ecd, radiochemical purity technology, applied in the directions of liquid transportation, radioactive carrier, radioactive physical shape, etc., can solve the problems of many impurities, high background, difficult clinical diagnosis, etc., and achieve the effect of clear imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment provides a two-step method with high radiochemical purity 99m The Tc-ECD marking process specifically includes the following steps:
[0031]S1. Preparation of stannous glucoheptonate sodium solution: Mix 5 μg of stannous chloride and 3.1 mg of sodium glucoheptonate, mix well, dissolve in 5 mL of 0.9% chloride at room temperature Add phosphate buffer to the sodium solution to adjust the pH of the solution to 5.5-6.0, and then prepare a stannous glucoheptonate sodium solution (ie, GH solution) for future use. Among them, the phosphate buffer is NaH 2 PO 4 and Na 2 HPO 4 Mixture of solutions, NaH 2 PO 4 NaH in solution 2 PO 4 Concentration is 0.2mol / L, Na 2 HPO 4 Na in solution 2 HPO 4 The concentration is 0.2mol / L, NaH in phosphate buffer 2 PO 4 solution and Na 2 HPO 4 The volume ratio of the solution is 12.3:87.7, so that the pH of the solution can be kept in the range of 5.5-6.9. In this embodiment, the pH of the solution is 6.3.
[0032...
Embodiment 2-7
[0035] The difference between Examples 2-7 and Example 1 is that the consumption of each raw material is different, see Table 1 for details, and the others are the same as Example 1.
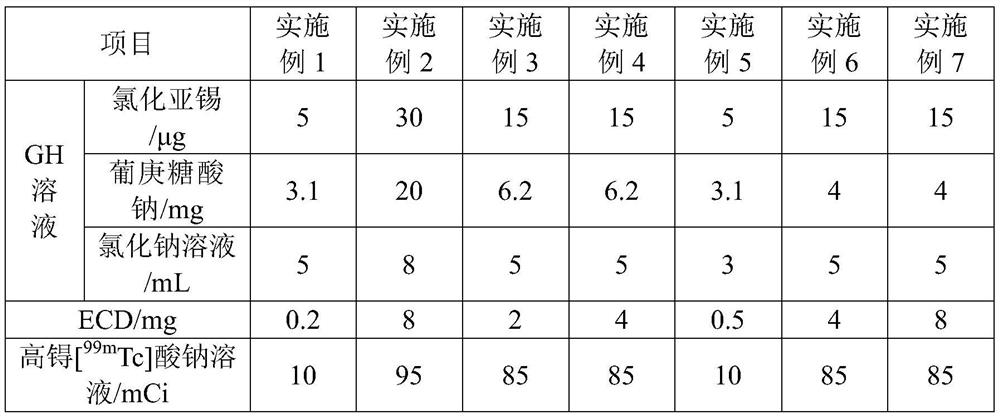
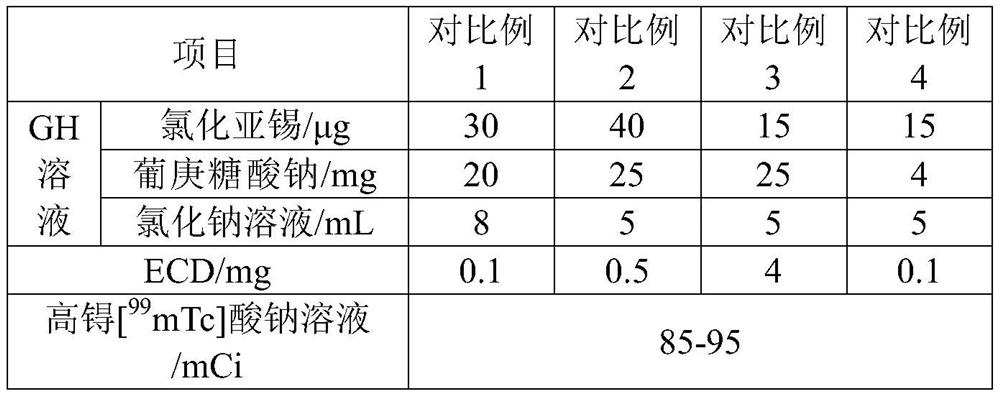
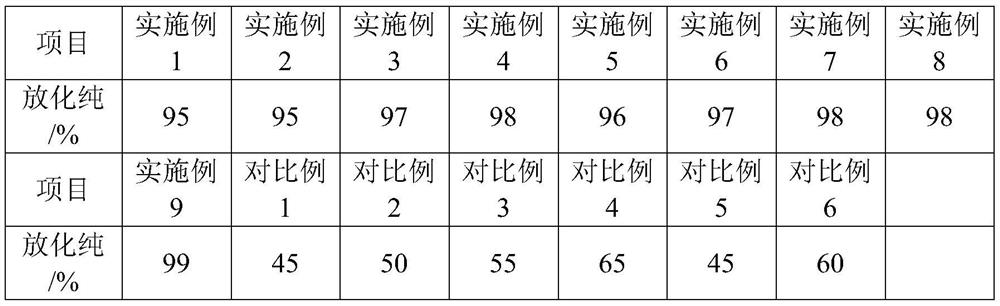
[0036] The preparation of table 1 embodiment 1-7 99m Raw material composition and dosage of Tc-ECD solution
[0037]
Embodiment 8
[0039] The difference between this example and Example 3 is that the reaction conditions in step S3 are different, specifically reacting at 90°C for 20 minutes to obtain the marker 99m Tc-ECD solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com