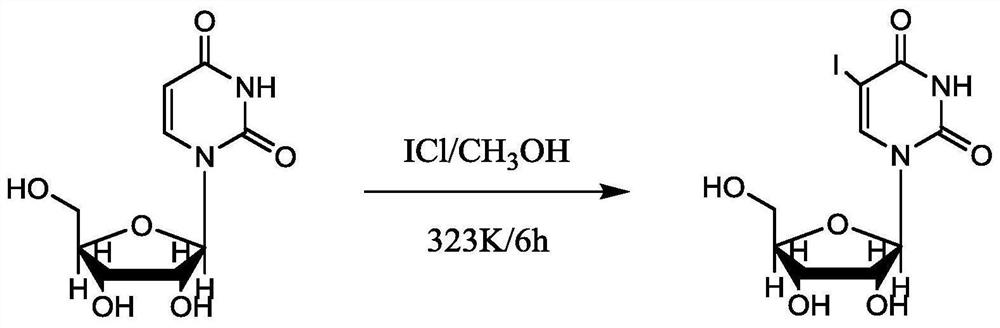
Synthesis method of 5-iodouracil nucleoside
A technology of iodouridine and uridine, which is applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of difficulty in recycling, high cost of synthetic methods, and high reaction risk factors, Achieve the effect of improving safety, high yield and improving safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A kind of synthetic method of 5-iodouridine of the present invention comprises the following steps: 1) get 10 parts of iodine monochloride and anhydrous methanol to dissolve in the ratio of 1:3, then add 50 parts of anhydrous methanol; 2 ) adding 10 parts of uridine, and raising the temperature of the system to 50°C, and stirring at a speed of 150rpm for 5h; 3) stopping heating and naturally cooling down to room temperature, filtering, washing with anhydrous methanol until white, and drying to obtain 5 - Iodouridine.
Embodiment 2
[0018] A kind of synthetic method of 5-iodouridine of the present invention comprises the following steps: 1) get 15 parts of iodine monochloride and anhydrous methanol to dissolve in the ratio of 1:5, then add 80 parts of anhydrous methanol; 2 ) adding 15 parts of uridine, and heating the system to 50°C, and stirring at a speed of 150rpm for 8h; 3) stopping heating and cooling down to room temperature naturally, filtering, washing with anhydrous methanol until white, and drying to obtain 5 - Iodouridine.
Embodiment 3
[0020] A kind of synthetic method of 5-iodouridine of the present invention comprises the following steps: 1) get 13 parts of iodine monochloride and anhydrous methanol to dissolve in the ratio of 1:4, then add 65 parts of anhydrous methanol; 2 ) adding 13 parts of uridine, and raising the temperature of the system to 50°C, and stirring at a speed of 150rpm for 7h; 3) stopping heating and cooling down to room temperature naturally, filtering, washing with anhydrous methanol until white, and drying to obtain 5 - Iodouridine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com