Compound extracted and separated from parthenocissus tricuspidata and application of compound in preparation of antidiabetic drugs
A compound and diabetes technology, applied in the field of preparation of anti-diabetic drugs, can solve problems such as complex components and unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
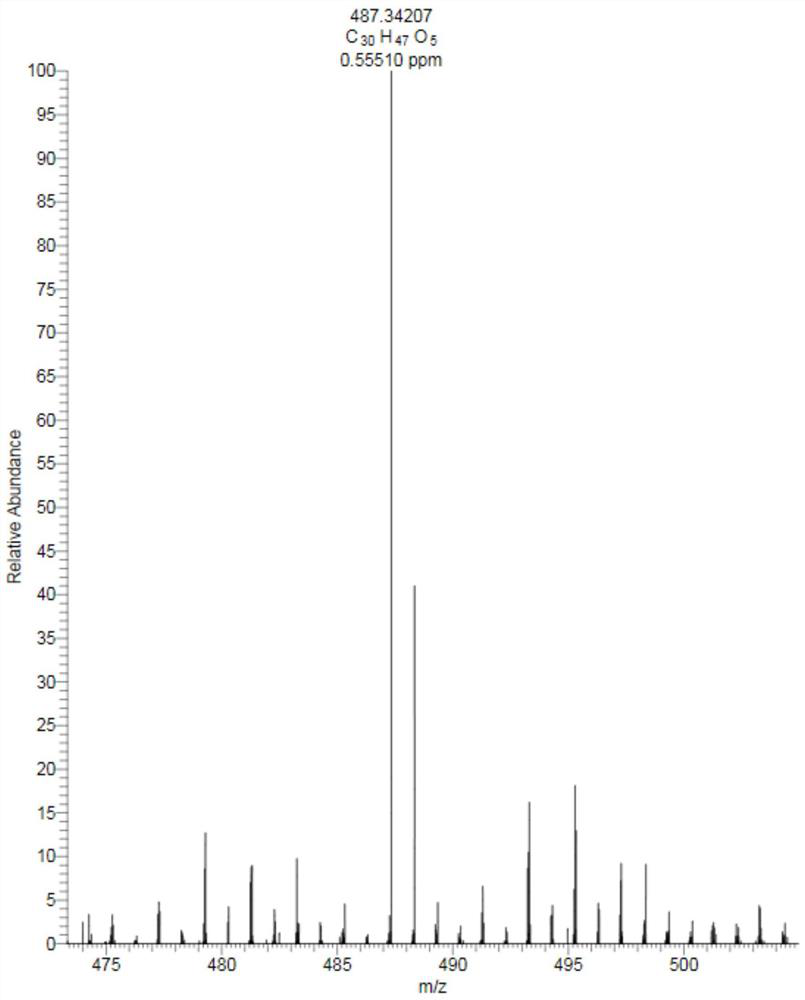
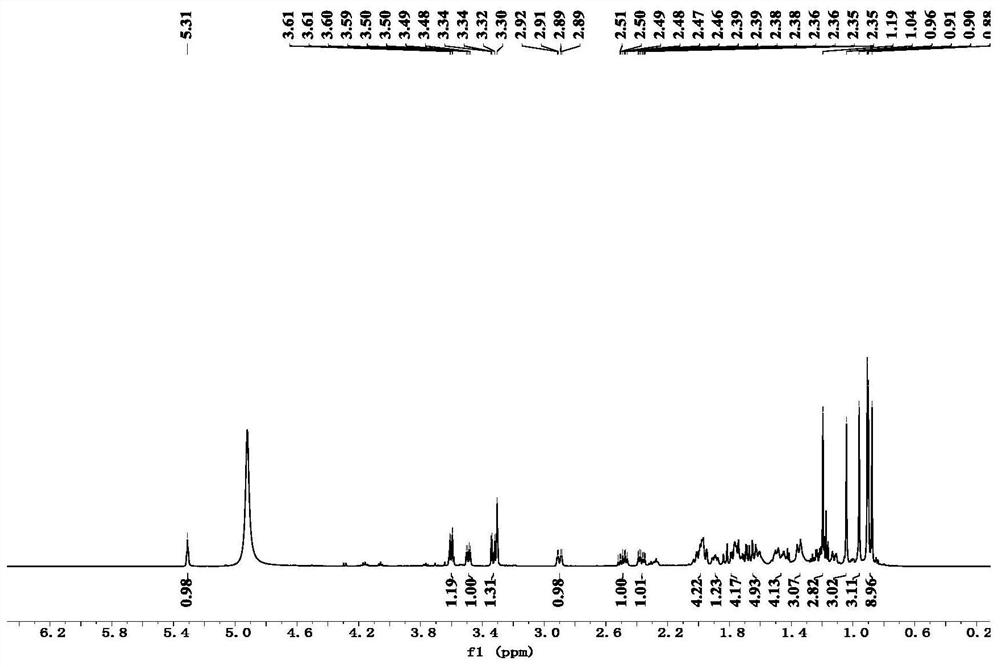
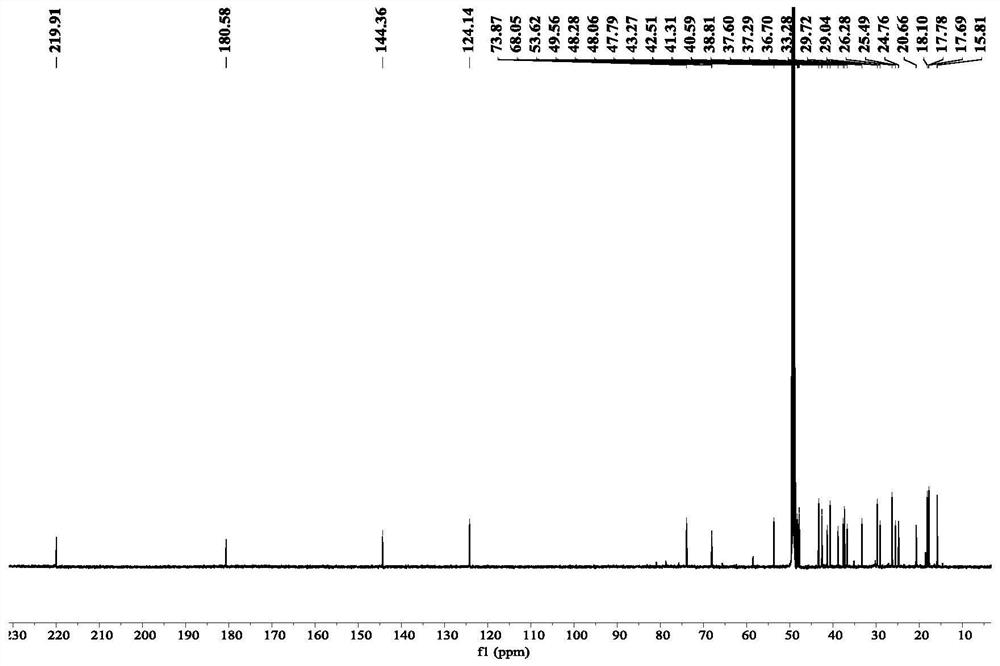
[0023] The preparation of embodiment 1 formula (1) compound
[0024] Step 1, get the root and rhizome 30.0kg of dry Caulophyllum robustum Maxim., after pulverizing through a 20 mesh sieve, use 95v / v% ethanol (120L) and 60v / v% ethanol (120L) successively Extract by rolling, combine the extracts, and concentrate to obtain 6.3kg of total extract;
[0025] Step 2, after suspending the total extract obtained in step 1 with water, extract with petroleum ether and ethyl acetate in sequence to obtain petroleum ether part extract (200.0g) and ethyl acetate part extract (410.0g);
[0026] Step 3, the ethyl acetate part extract (410.0g) obtained in step 2 is used for silica gel column chromatography, and gradient elution is carried out with petroleum ether: ethyl acetate system, and the volume ratios are: 10:1, 8:1, 6:1, 4:1, 2:1, 1:1, 0:1), collect petroleum ether: ethyl acetate volume ratio 2:1-1:1 eluate, numbered as Fr.C, after subtraction Compress and concentrate to dryness, set a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com