cd47 antagonists and uses thereof
A CDR1-CDR3, antigen technology, applied in the field of biomedicine or biopharmaceuticals, can solve problems such as insufficient anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1. Generation of anti-CD47 monoclonal antibodies
[0064] 1.1 Immunization program
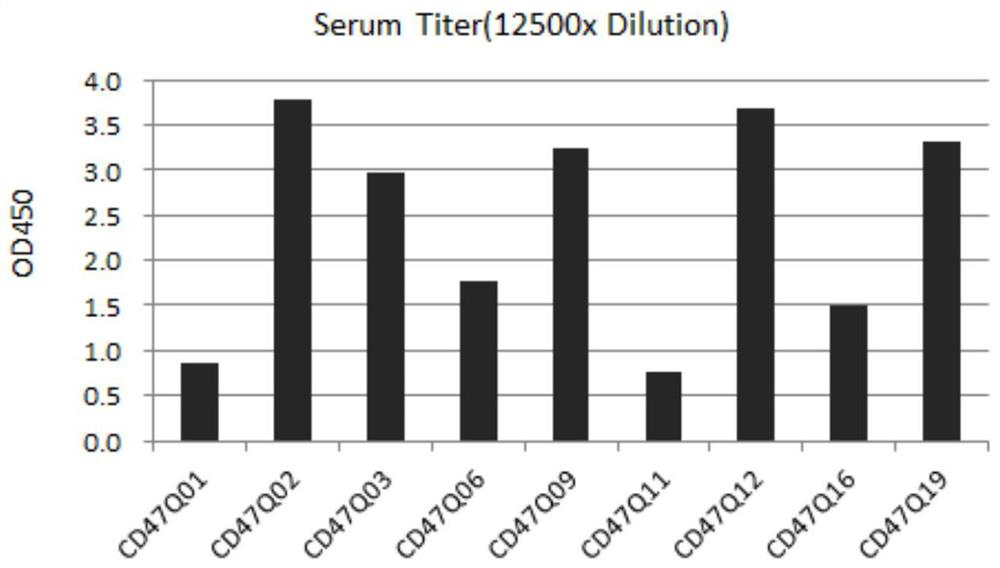
[0065] BoAn-hMab1 transgenic mice with Boan human antibody were immunized with CD47-His (ACROBiosystems, cat. no. CD7-H5227) or CD47-mFc (ACROBiosystems, cat. no. CD7-H52A5) emulsified with Freund's adjuvant. Freund's complete adjuvant was used for the first immunization, and Freund's incomplete adjuvant was used for the second to sixth immunization. A total of 20 mice were immunized this time, and serum titers were detected by Elisa. Coat the protein CD47-His (ACROBiosystems, catalog number CD7-H5227) with a concentration of 1ug / ml with CBS coating solution (pH9.6 carbonate solution), 100ul / well overnight at 4 degrees; block with 3% skimmed milk powder at 37 degrees for 1h ; Dilute the serum to 100X, 500X, 2500X, 12500X with PBST, add 100ul to each well. Incubate at 37°C for 1h; then add HRP-goat anti-human antibody (474-1006, KPL), incubate at 37°C for 1h, after color devel...
Embodiment 2
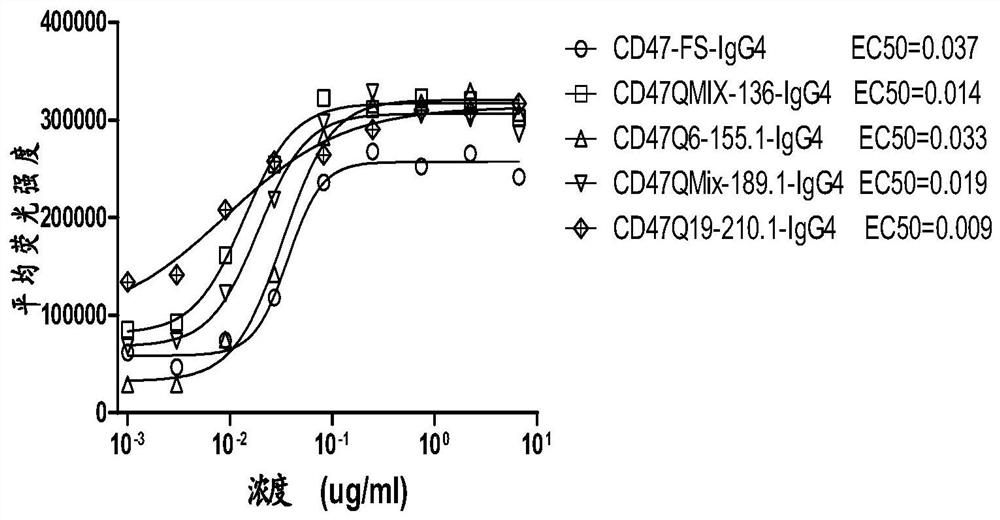
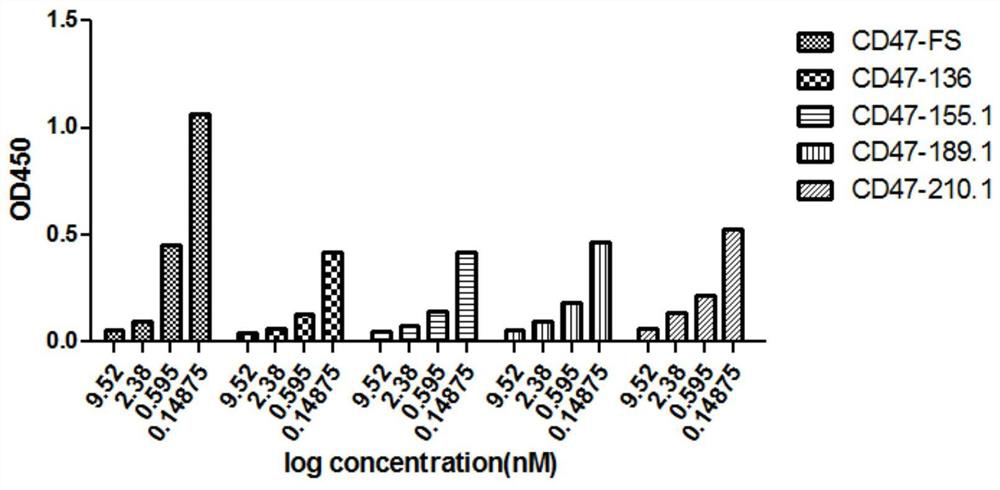
[0081] Example 2. Molecular construction and production of blocking antibodies
[0082] The clones CD47QMix-136\189, CD47Q6-155, CD47Q19-210 (hereinafter referred to as clone "136 / 189 / 155 / 210") were sent to Invitrogen Biotechnology Co., Ltd. for sequencing. The sequences are shown in Table 2.
[0083] Table 2. Amino acid sequences of candidate clones
[0084]
[0085]
[0086] Sequence analysis found that clone 155, clone 189 and clone 210 had N-glycosylation sites, and primers were designed to mutate them to obtain clones 155.1, 189.1 and 210.1, respectively. The heavy chain of clone 155.1 belongs to the germline gene IGHV4-34 family by sequence analysis, and the light chain belongs to the germline gene IGKV4-1 family. The amino acid sequence of each clone is as follows in Table 3:
[0087] Table 3. Amino acid sequences of candidate clones
[0088]
[0089] The antibody variable region gene was amplified by conventional molecular biology PCR (2*Phanta Max Master M...
Embodiment 3
[0099] Example 3. Characterization of Candidate Antibodies
[0100] 3.1 Forte Bio detects the affinity of candidate antibodies to CD47 protein
[0101] Antibody binding kinetics were measured using an OctetRED 96 instrument based on Biolayer Interferometry BLI. The antibody (10 μg / mL) was coupled to PROA Biosensors, the loading height was 1 nm, and the CD47 was serially diluted 2-fold with PBST, starting at 25 nM, and set to 0 concentration, the Association time was set to 300s, and the Dissociation time was set to 300s. After the detection, the binding constant (kon) and the dissociation constant (kdis) were calculated using Curve Fitting of 1:1 Model, and the equilibrium dissociation constant (kD) was calculated as the ratio kd / ka. The results are shown in Table 4. Compared with the control antibody, the affinity of the four candidate antibodies is higher than that of the control antibody except that the KD of CD47Q19-210.1-IgG4 is similar to that of the control antibody. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com