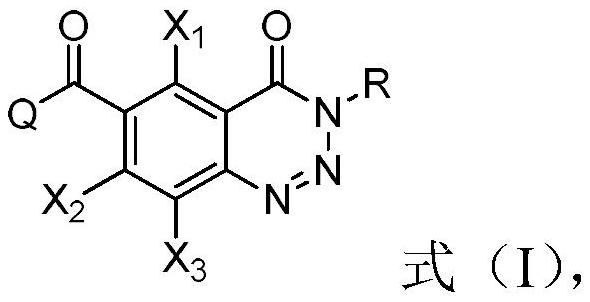
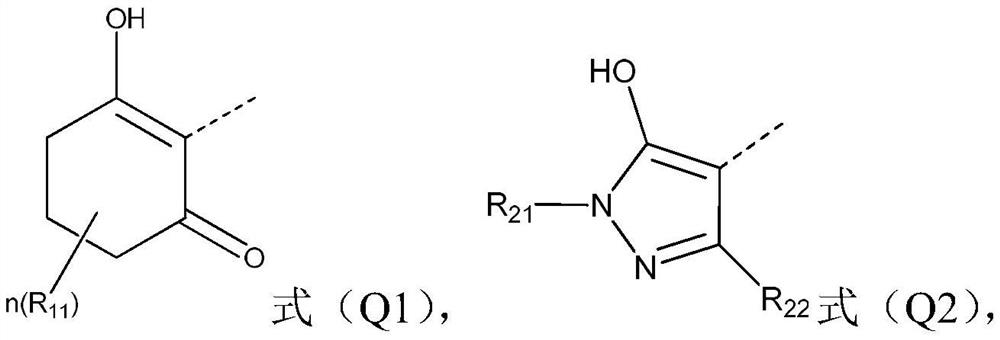
Compound containing benzotriazinone structure, preparation method and application thereof, and herbicide
A technology of benzotriazinone and compounds, which is applied in the field of herbicides, can solve the problems of increased herbicide dosage in wheat fields, excessive pesticide residues, and increased control costs, and achieve excellent herbicidal activity, good safety, and excellent HPPD inhibitory activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0085] Preparation Example 1: Preparation of Compound 1
[0086]
[0087] Add 75.5g of the compound shown in 1-1 to a 2L reaction flask at room temperature, add 1L of glacial acetic acid while stirring, then dissolve 88.5g of ICl into 300mL of glacial acetic acid, and add dropwise to the above reaction within 30min while stirring In the system, after the dropwise addition was completed, the stirring reaction was continued for about 3 hours. After the reaction was completed, the reaction solution was suction-filtered, and the obtained solid was washed with 500 mL of glacial acetic acid and dried to obtain Intermediate 1-2 with a yield of 98%.
[0088] Add 135g of intermediate 1-2 to a 2L flask, add 1L of tetrahydrofuran, then add 145.5g of triphosgene, then react overnight, wash with saturated sodium carbonate solution after the reaction, extract with ethyl acetate, combine the organic phases, anhydrous Drying over sodium sulfate and dehydration gave Intermediate 1-3 with a...
preparation example 2
[0096] Preparation Example 2: Preparation of Compound 50
[0097]
[0098] Add 5.7g of intermediate 1-5 into a 200mL single-neck flask, add 50mL of DMF, and add 7.2g of Cs under stirring 2 CO 3 The stirring reaction was continued for about 30 min. Then 3.7 g of benzyl bromide was slowly added dropwise into the reaction system, and after the dropwise addition was completed, the reaction was stirred at room temperature overnight. After the reaction was completed, 100 mL of water was added to the system, and the reaction system was extracted 3 times with 50 mL of ethyl acetate each time. The organic layers were combined and dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain intermediate 1-11 in a yield of 90%.
[0099] 6.8 g of Intermediate 1-11, 3.2 g of CuCN were added to a 200 mL two-neck flask, and 60 mL of dry DMF was added. The reaction was refluxed for 12 hours. After the reaction was completed, DMF was distilled off un...
preparation example 3
[0103] Preparation Example 3: Preparation of Compound 77
[0104]
[0105] Add 885 mg of intermediate 1-13 to a 50 mL single-necked bottle, add 15 mL of dry THF, and slowly add 714 mg of SOCl dropwise at room temperature 2 , After the dropwise addition was completed, the reaction was refluxed at 75°C for about 1.5h, and the reaction progress was tracked by TLC, and the solvent was removed after the reaction. Add 20 mL of dry CH 2 Cl 2 , 378 mg of compound 1-9b, 606 mg of Et 3 N, reacted for about 0.5h, followed by TLC until the acid chloride disappeared. After the reaction is completed, wash once with 20 mL of water, wash twice with 10 mL of 1 mol / L HCl each time, and wash twice with 10 mL of saturated NaHCO each time. 3 Wash 2 times, anhydrous Na 2 SO 4 Drying and passing through the column gave intermediate 1-15 with a yield of 90%.
[0106] 1.1g of intermediate 1-15 was added to a 50mL two-necked bottle, 25mL of anhydrous acetonitrile was added, and N 2 Add 545 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com