Preparation method of polyimide and application of water-based lithium ion battery thereof
A polyimide and compound technology, applied in the field of polymer preparation, can solve the problems of unsuitability for large-scale production, complex preparation methods, low yield, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation of embodiment 1 polyimide
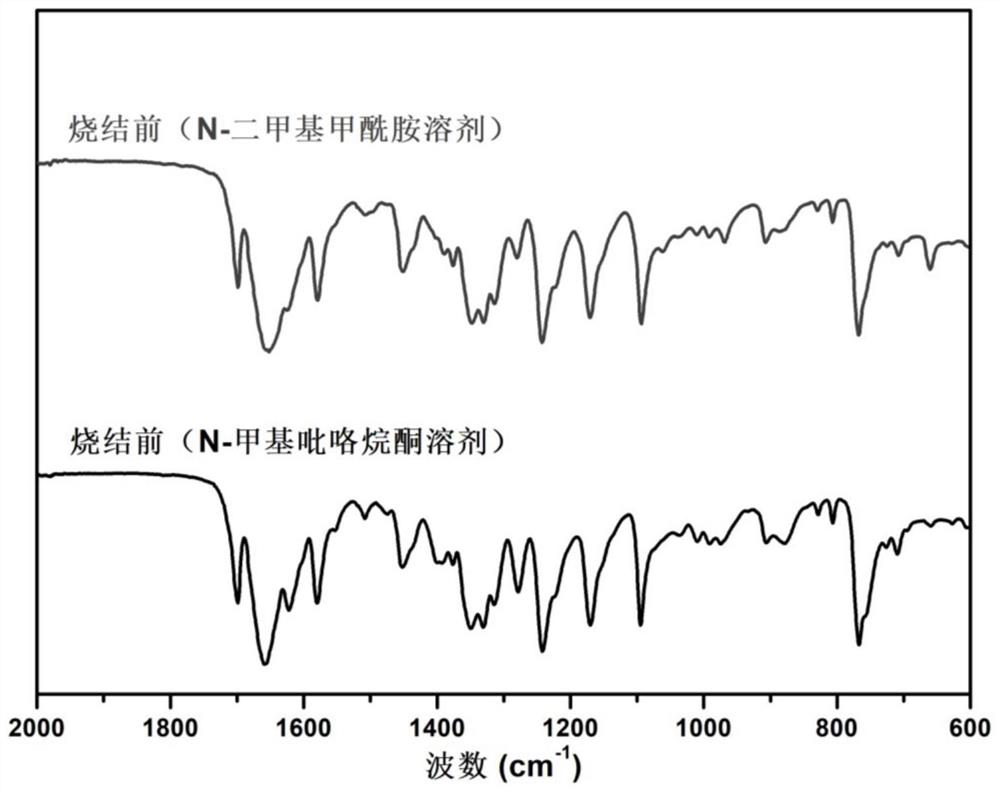
[0082] Step A: Add 1,4,5,8-naphthalene tetracarboxylic anhydride (NTCDA) powder and ethylenediamine (EDA) solution in the N-dimethylformamide (DMF) solution at a molar ratio of 1:1, keeping Magnetic stirring. The reaction concentration is 50g / 500ml. The reaction vessel is a glass flask. The flask was protected with argon. The flask was transferred to a 50°C oil bath, and the temperature was gradually raised to 150°C. The reaction was maintained at this temperature for 4 hours.
[0083] Step B: collect and centrifuge the products obtained by using method A and method B, and remove the supernatant. For the centrifuged sediment below, it was diluted and washed with N-dimethylformamide, and a second centrifugation was performed. Wash and centrifuge with ethanol. The final material was dried in a vacuum oven at 80° C. for 4 hours to obtain a dry powder, which was designated as polyimide sample 1.
Embodiment 2
[0084] The preparation of embodiment 2 polyimide
[0085] Step A: Add 1,4,5,8-naphthalene tetracarboxylic anhydride (NTCDA) powder and ethylenediamine solution (EDA) into the N-methylpyrrolidone (NMP) solution at a molar ratio of 1:1, and keep magnetic stirring . The reaction concentration is 50g / 500ml. The reaction vessel is a glass flask. The flask was protected with argon. The flask was transferred to a 50°C oil bath, and the temperature was gradually raised to 150°C. The reaction was maintained at this temperature for 4 hours.
[0086] Step B: collect and centrifuge the products obtained by using method A and method B, and remove the supernatant. For the centrifuged sediment below, dilute and wash with N-methylpyrrolidone, and perform a second centrifugation. Wash and centrifuge with ethanol. The final material was dried in a vacuum oven at 80° C. for 4 hours to obtain a dry powder, which was designated as polyimide sample 2.
Embodiment 3
[0087] The preparation of embodiment 3 polyimide
[0088] Step A: Add 1,4,5,8-naphthalene tetracarboxylic anhydride (NTCDA) powder and ethylenediamine solution (EDA) into the N-methylpyrrolidone (NMP) solution at a molar ratio of 1:1, and keep magnetic stirring . The reaction concentration is 50g / 300ml. The reaction vessel is a glass flask. The flask was protected with argon. The flask was transferred to a 50°C oil bath, and the temperature was gradually raised to 150°C. The reaction was maintained at this temperature for 6 hours.
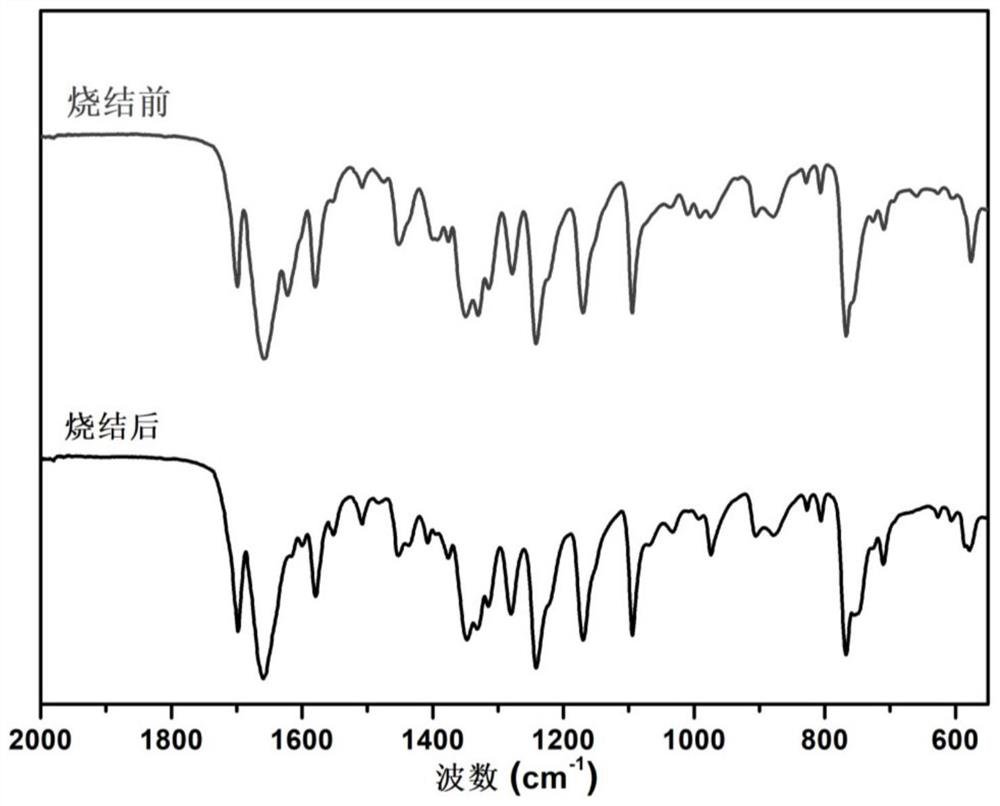
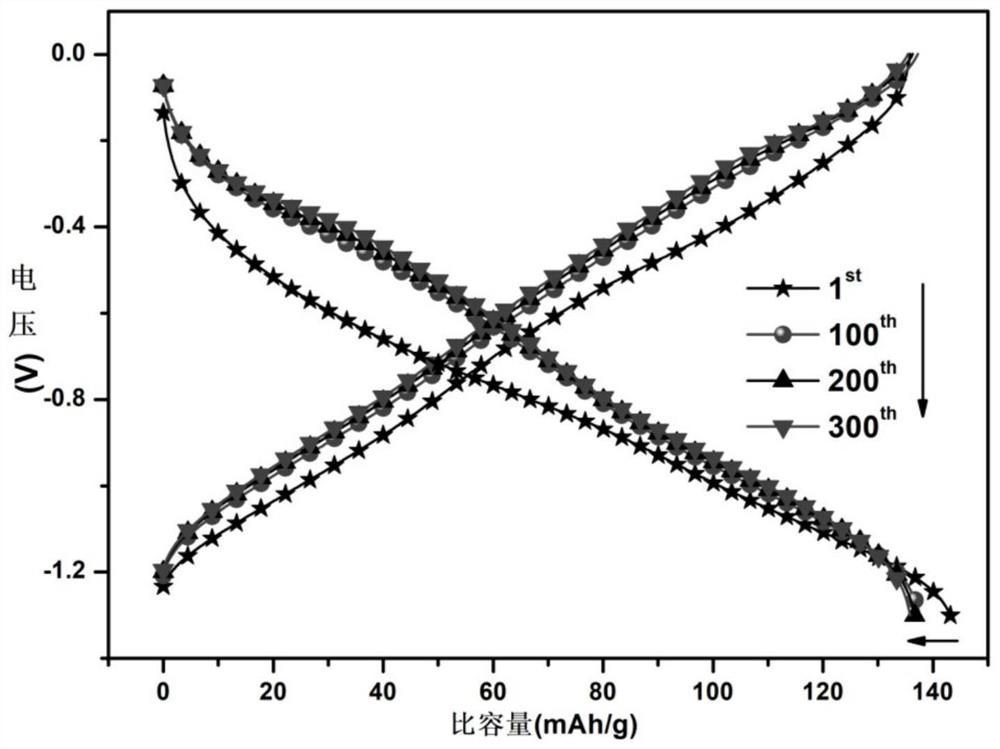
[0089] Step B: collect and centrifuge the products obtained by using method A and method B, and remove the supernatant. For the centrifuged sediment below, dilute and wash with N-methylpyrrolidone, and perform a second centrifugation. Wash and centrifuge with ethanol. The final material was dried in a vacuum oven at 80° C. for 4 hours to obtain a dry powder, which was sintered at 350° C. for 8 hours under the protection of argon, which was r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com