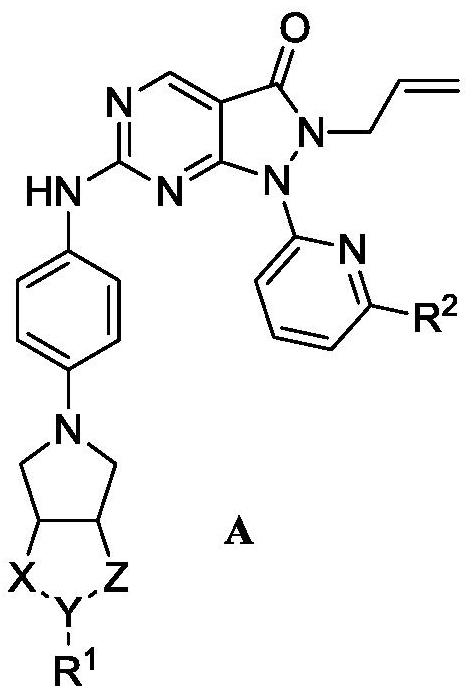
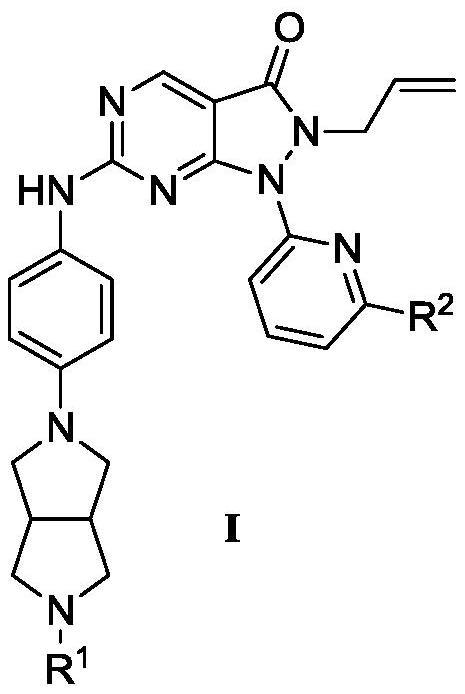
Pyrazolone-fused pyrimidine compound as well as preparation method and application thereof
A technology of pyrazolone and pyrimidine, applied in the field of pyrazolo pyrimidine compounds, can solve problems such as single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and Embodiment 39
[0560]
[0561] first step:
[0562] Compound (I-1-a)(3aR, 6aS)-2,3,3a,4,6,6a-hexahydro-1H-pyrrolo[3,4-c]pyrrole-5-carboxylic acid tert-butyl ester (2000mg, 9.4211mmol) was dissolved in DMSO (30mL), then potassium carbonate (3eq, 28.263mmol) and 1-fluoro-4-nitro-benzene (I-1-b) (1eq, 9.4211mmol) were added, and The reaction was heated to 120°C and stirred for 2 hours. The reaction was poured into 5ml of water and a yellow solid formed. The solid was filtered, washed with water (2×5ml), collected and dried in vacuo to obtain compound (I-1-c) tert-butyl (3aR, 6aS)-2-(4-nitrophenyl)-1,3 , 3a,4,6,6a-tert-butyl hexahydropyrrolo[3,4-c]pyrrole-5-carboxylate (3g, 8.998mmol), yield 95.51%, yellow solid. LC-MS: m / z: (M+H-tBu) + = 278.2.
[0563] Step two:
[0564] Compound (I-1-c)(3aR, 6aS)-2-(4-nitrophenyl)-1,3,3a,4,6,6a-hexahydropyrrolo[3,4-c]pyrrole - Tert-butyl 5-carboxylate (0.4g, 1mmol) was dissolved in ethanol (70mL) and THF (10mL) solution, then Pd / C (0.1eq, 0.1mmol) a...
Embodiment 2
[0570]
[0571] Compound (I-1) 6-[4-[(3aR, 6aS)-2,3,3a, 4,6,6a-hexahydro-1H-pyrrolo[3,4-c]pyrrol-5-yl ]anilino]-2-allyl-1-[6-(1-hydroxy-1-methyl-ethyl)-2-pyridyl]pyrazolo[3,4-d]pyrimidin-3-one (110mg, 0.2146mmol) was dissolved in methanol solution (6mL), then added formaldehyde (10eq, 2.146mmol, 37%) and glacial acetic acid (0.2mL), and the reaction was stirred at room temperature for 5 minutes. Then, sodium acetate borohydride (2eq, 0.4292mmol) was added thereto, and the reaction solution was stirred and reacted at room temperature for 12 hours. NaHCO for reaction 3 The aqueous solution was quenched and extracted with ethyl acetate (2×20 mL), the organic layer was washed with brine (1×20 mL), and washed with Na 2 SO 4 Dry, filter and concentrate to give crude product. The crude product was further purified by Pre-HPLC with CH 3 CN / water (0.1% HCOOH) was eluted from 20% to 50% to give compound (I-2) 6-[4-[(3aR,6aS)-2-methyl-1,3,3a,4,6 ,6a-Hexahydrohexahydropyrrolo[3,4...
Embodiment 15
[0573]
[0574] first step:
[0575] Dissolve 1 g of tert-butyl(3aR,6aS)-5-(4-nitrophenyl)hexahydropyrrole[3,4-c]pyrrole-2(1H)-carboxylate (I-1-c) in 10 ml of trifluoroacetic acid was added to 40 ml of dichloromethane, reacted at room temperature for 1 h, and the reaction solution was spin-dried to obtain 0.7 g of yellow solid (I-15-a), with a yield of 91%. LC-MS: m / z: (M+H) + = 234.1.
[0576] Step two:
[0577] Dissolve (3aR,6aS)-2-(4-nitrophenyl)octahydropyrrole[3,4-c]pyrrole (I-15-a) (200mg, 0.26mmol) in 15ml of acetonitrile and add potassium carbonate (170 mg, 1.72 mmol) and bromoacetonitrile (I-15-b) (124 mg, 1 mmol). After reacting at room temperature for 18 hours, it was concentrated and then extracted with 30ml of dichloromethane and 10ml of water. Thin layer chromatography (DCM / CH 3 OH=10 / 1) was purified to obtain 40 mg of a yellow solid (I-15-c), with a yield of 56%. LC-MS: m / z: (M+H) + = 273.1.
[0578] third step:
[0579] 2-((3AR, 6AS)-5-(4-nitrophen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com