Antibody fusion protein targeting Frizzled-7 as well as preparation method and application of antibody fusion protein
A fusion protein and antibody technology, applied in the field of bioengineering, can solve the problems of patient antibody response difference, decline, and different affinity, and achieve the effects of preventing tumor immune escape, enhancing killing effect, and inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
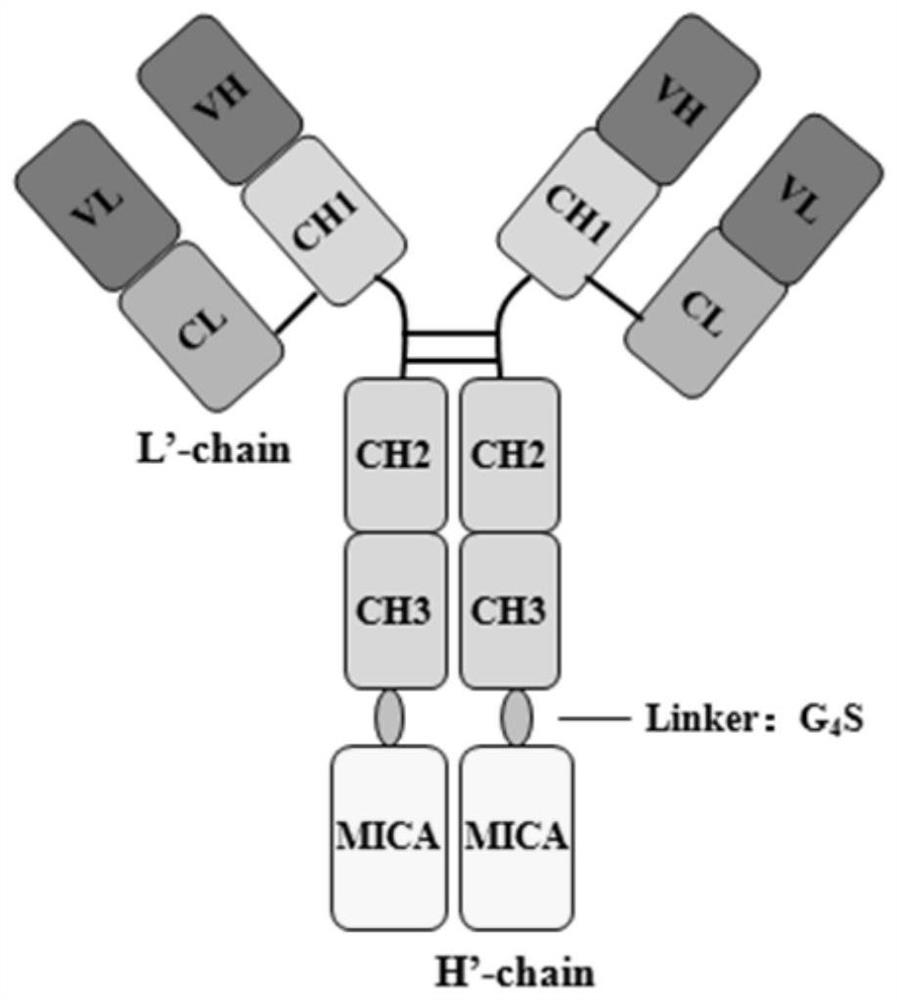
[0050] Example 1: Construction of fusion protein SHH002-hu1-MICA
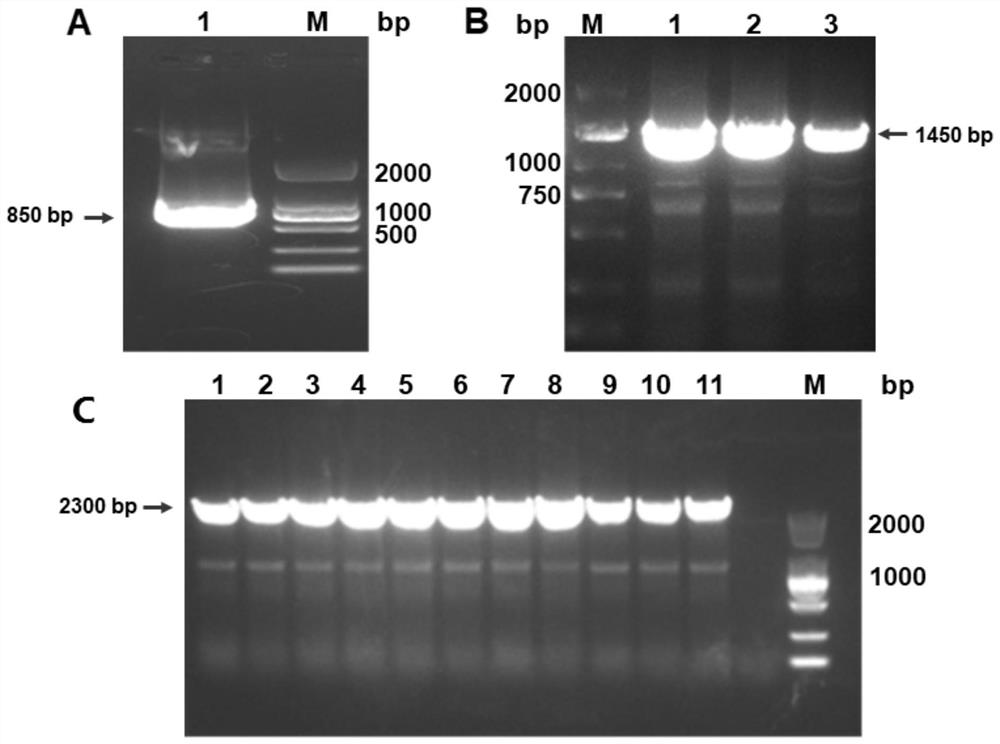
[0051] First, obtain the gene sequence (GeneID: 100507436, protein: Q29983) encoding human MICA protein α1, α2 and α3 extracellular domains from genebank, use it as a template, design primers, forward primer MICA-F: GAGCCTGTCTCCTGGCAAAGGAGGTG, reverse Primer pcDNA3.4-3R: GTTGATTGTCGAGATATCAAATTATC, PCR amplifies the MICA' fragment. The heavy chain construction of the fusion protein SHH002-hu1-MICA takes the SHH002-hu1 heavy chain gene H' and MICA' gene as templates, and performs overlapping PCR extension amplification to obtain the complete H'-MICA gene. Forward primer insert-F: GCTACGCGTGTCCACTCC , reverse primer IgG1-3R: TTTGCCAGGAGACAGGCTCAGGGACTTC. The PCR product was detected by 1.0% agarose gel electrophoresis, and the target gene was recovered by the agarose gel extraction kit. The final product of PCR amplification and the vector pcDNA3.4 were double-digested with restriction endonucleases. After the ...
Embodiment 2
[0053] Example 2: Expression, purification and identification of fusion protein SHH002-hu1-MICA
[0054] The recombinant plasmids H'-MICA-pcDNA3.4 and L-pcDNA3.4 were transiently transfected into EXPICHO-S cells with liposome transfection reagent, and the serum-free medium was replaced for protein expression.
[0055] 1. Take the cell culture supernatant, centrifuge at 8000rpm for 15min, filter the sample with a 0.22μm filter membrane and purify it with a Protein A column. Elute with 100 mM glycine buffer (pH 3.5, pH 2.7), collect the eluted peaks; and neutralize the collection with 1M Tris buffer (pH 9.0). The purified protein was identified by SDS-PAGE electrophoresis under non-reducing and reducing conditions, respectively, to identify the molecular weight of SHH002-hu1-MICA.
[0056] 2. Using Agilent HPLC 1100 instrument to further verify the purity of the purified fusion protein SHH002-hu1-MICA by SEC-HPLC method. The selected molecular exclusion chromatography column i...
Embodiment 3
[0066] Example 3: BLI experiment of fusion protein SHH002-hu1-MICA
[0067] The affinity of SHH002-hu1-MICA to rhFzd7 protein and rhNKG2D-Fc protein was verified by BLI method using protein interaction instrument Fortebio Octet Red96.
[0068] 1. Solution preparation: PBST buffer: 0.025% Tween 20 was dissolved in PBS with pH 7.2; stationary phase working solution: SHH002-hu1-MICA / SHH002-hu1 was prepared with PBST buffer to 100nM; rhFzd7 working solution: rhFzd7 was prepared with PBST buffer 1000nM, 500nM, 250nM, 125nM, 62.5nM, 31.25nM and 15.625nM; rhNKG2D working solution: rhNKG2D-Fc was prepared in PBST buffer to 300nM, 100nM and 33nM.
[0069] 2. Operation process: Open the Fortebio instrument and related software, and select the Advance Kientics experimental mode. First, the Anti-Human Fab-CH1 2nd Generation (FAB2G) Sensor was used to capture SHH002-hu1-MICA / SHH002-hu1, and then the captured fusion protein / parental antibody was used to bind different concentrations of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com