Application of lactobacillus reuteri in preparation of medicine for treating acute hepatic failure (AHF)
A technology of Lactobacillus reuteri and acute liver failure, which is applied in the field of application of Lactobacillus reuteri in the preparation of drugs for the treatment of acute liver failure. Liver inflammation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] 1. Experimental grouping and treatment:
[0030] Grouping of rats: divided into 3 groups, respectively, Lactobacillus reuteri supplementation group (that is, Lactobacillus reuteri, and galactosamine model), positive control group (that is, the same amount of PBS, and galactose amine modeling), blank control group (no probiotics, no galactosamine modeling).
[0031] Lactobacillus reuteri gavage: Dissolve Lactobacillus reuteri in sterile PBS solution, and give 1*10E9 CFU / ml / cause / day. The model group was administered by gavage continuously for 14 days. At the same time, the control group was given PBS solution for 14 days.
[0032] Induction of liver failure: After fasting for 12 hours, intraperitoneal injection of 1.1 g / kg body weight of galactosamine was given to the control group and the model group respectively to induce liver failure.
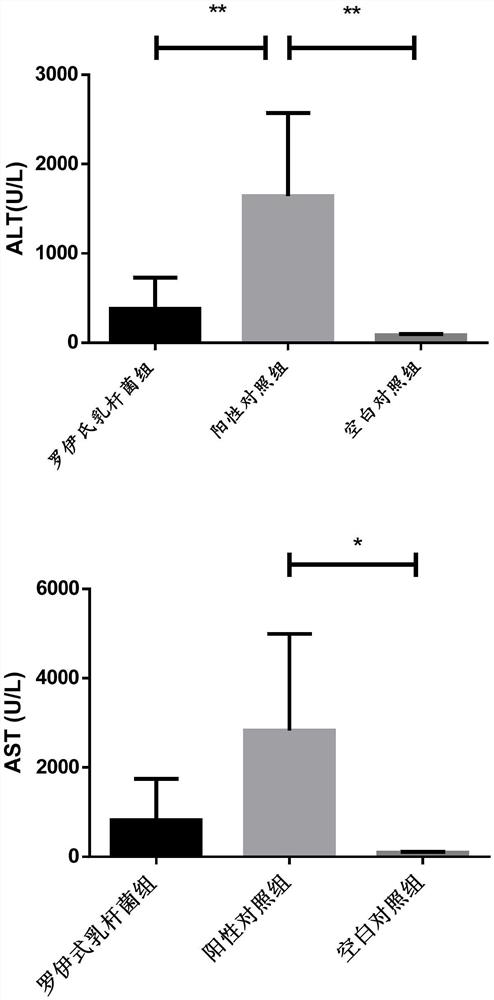
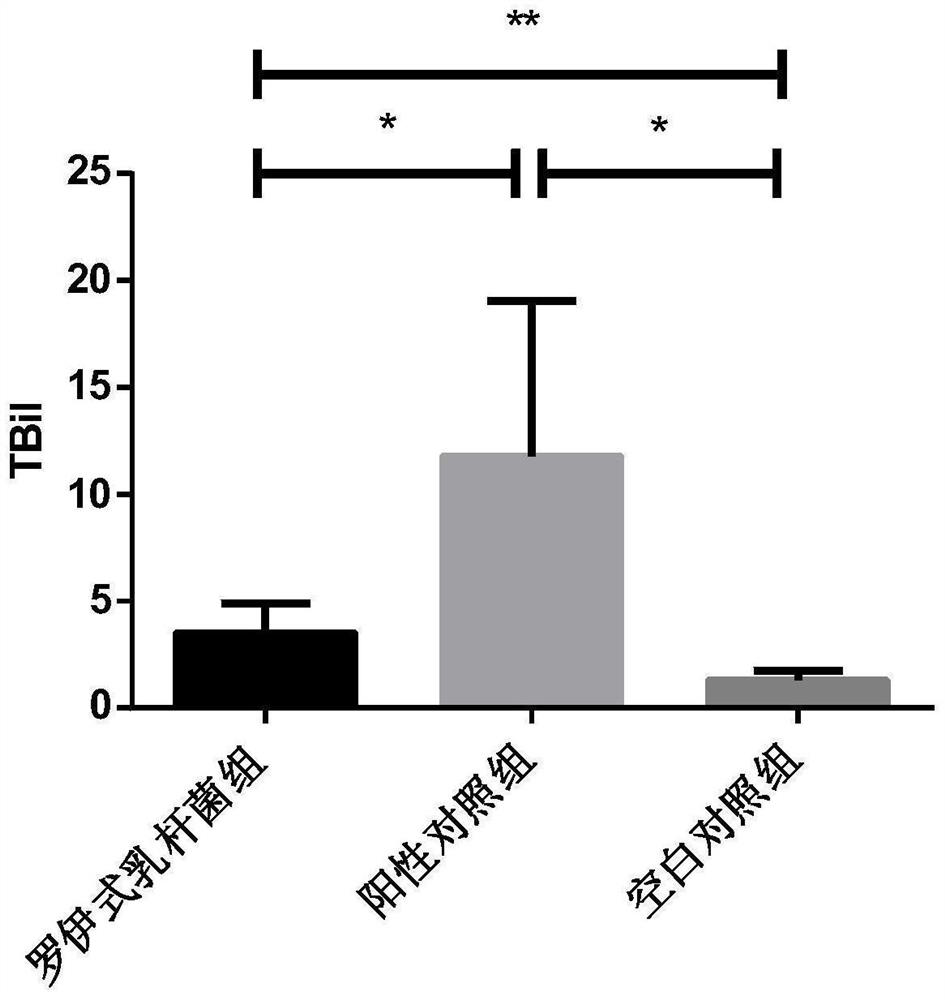
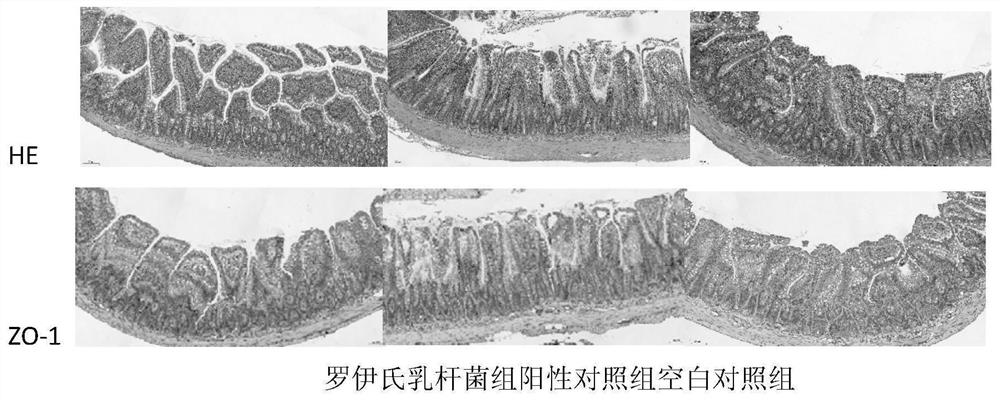
[0033] 2. Improvement effect of Lactobacillus reuteri on liver inflammation in rats with galactosamine-induced acute liver failur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com