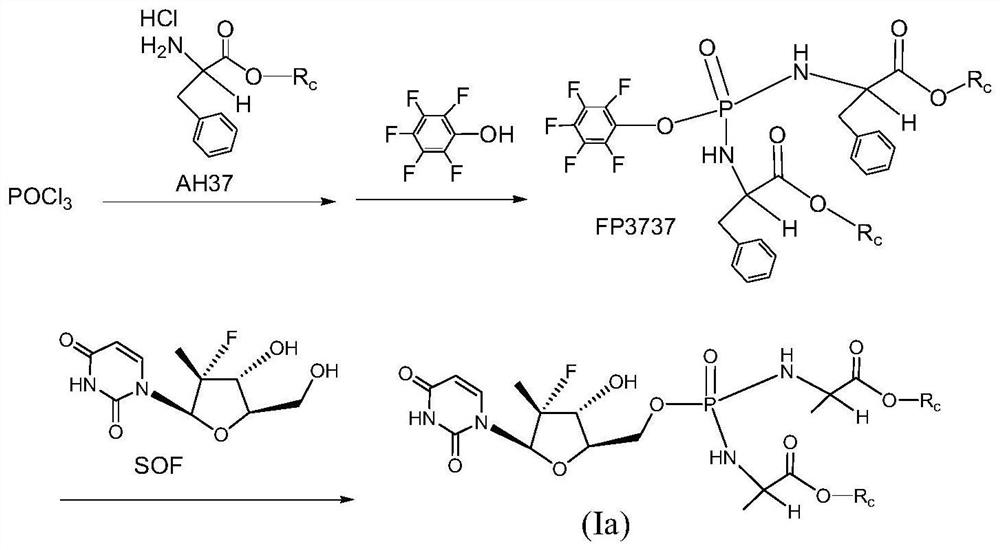
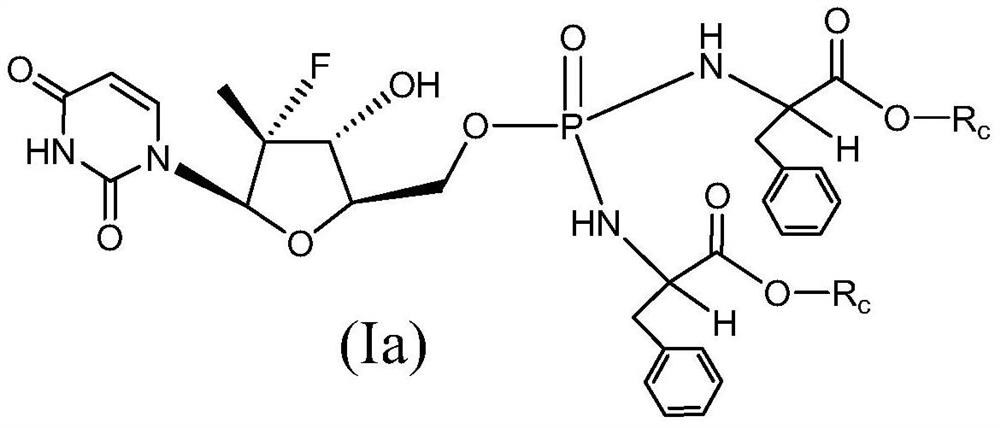
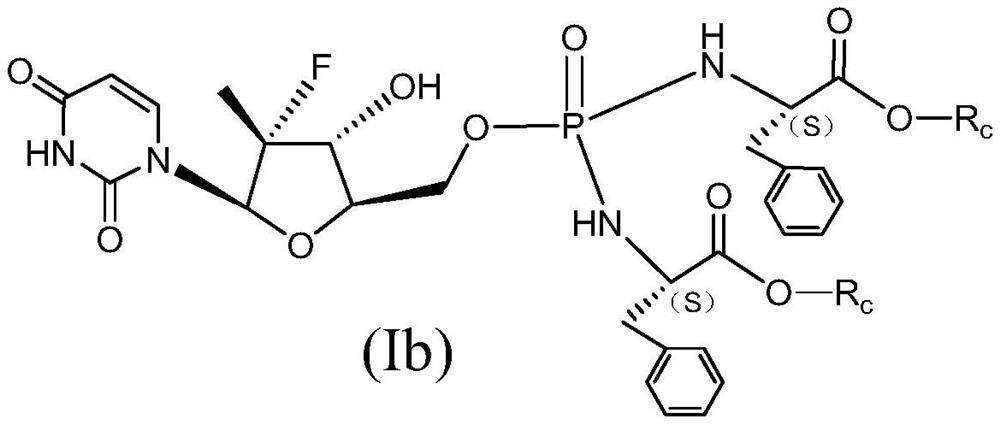
Uridine bisphenylpropionate-based phosphoramidate compound, its pharmaceutical composition, its preparation method and application
A technology based on phosphoramidate and bisphenylpropionate, applied in the field of medicine, can solve the problems of large dosage, low bioavailability, and long-term safety of virus drug resistance, and achieve the effect of inhibiting infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] N-[(Pentafluorophenoxy)(((S)-1-(isopropoxycarbonyl)-2-phenylethyl)amino)phosphoryl]-L-phenylalanine isopropyl ester (FP370370 )Synthesis.
[0040]
[0041] Phosphorus oxychloride (5g, 3.04ml, 32.6mmol) was added to the reaction flask, 200mL of acetonitrile was added, cooled to -70°C, HA370 (14.331g, 58.8mmol) was slowly added, cooled to -70°C, and triethyl was added dropwise. 60 mL of acetonitrile solution of amine (7.3g, 10ml, 72mmol) was added, warmed to 0°C, and reacted for 3 hours. A solution of pentafluorophenol (5.4g, 29.4mmol) and triethylamine (7.3g, 10ml, 72mmol) in acetonitrile 60 mL of the solution was added dropwise to the above solution, stirred at 0°C for 1 hour, raised to room temperature, and stirred overnight, then 100 mL of dichloromethane and 100 mL of water were added, the organic phase was separated, dried with anhydrous sodium sulfate, and then reduced in pressure. Concentrated and the residue was separated on a silica gel column (0-30% ethyl a...
Embodiment 2
[0044] N-[(Pentafluorophenoxy)(((S)-1-(ethoxycarbonyl)-2-phenylethyl)amino)phosphoryl]-L-phenylalanine ethyl ester (FP371371) synthesis.
[0045]
[0046] FP371371 was synthesized by a similar synthesis method in Example 1.
[0047] H NMR data of FP371371: 1H NMR (400MHz, CDCl 3 )δ(ppm): 1.17-1.36(6H, m, 2×CH 3 ), 3.75-3.94 (8H, m, 2×CH 2 , 2×NH and 2×NCH), 4.12-4.40(4H, m, 2×COOCH 2 ), 7.05-7.38 (10H, m, hydrogen on two benzene rings).
[0048] LCMS-ESI + (m / z): 615.5 (M+H).
Embodiment 3
[0050] N-[(Pentafluorophenoxy)(((S)-1-(isobutoxycarbonyl)-2-phenylethyl)amino)phosphoryl]-L-phenylalanine isobutyl ester (FP372372 )Synthesis.
[0051]
[0052] FP372372 was synthesized by the similar synthesis method of Example 1.
[0053] H NMR data of FP372372: 1 H NMR (400MHz, CDCl 3 )δ(ppm): 0.86-1.15 (12H, m, 4×CH 3 ), 2.34-2.50 (2H, m, 2×CH), 3.76-3.95 (8H, m, 2×CH) 2 , 2×NH and 2×NCH), 4.04-4.39 (4H, m, 2×COOCH) 2 ), 7.07-7.38 (10H, m, hydrogen on two benzene rings). LCMS-ESI + (m / z): 671.6 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com