Composition for preventing virus infection comprising poly-gamma-glutamic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of γ-PGA Having Ultra High Molecular Weight and Measurement of Molecular Weight Thereof
[0029]A 5 L fermenter containing a 3 L basal medium for γ-PGA production (GS medium containing 5% L-glutamic acid: 5% glucose, 1% (NH4)2SO4, 0.27% KH2PO4, 0.42% Na2HPO4.12H2O, 0.05% NaCl, 0.3% MgSO4.7H2O, pH 6.8) was inoculated with 1% culture broth of Bacillus subtilis var chungkookjang (KCTC 0697BP) and then cultured at a stirring speed of 150 rpm, an air injection rate of 1 vvm and a temperature of 37° C. for 72 hours. Cells were removed from the culture broth after completion of the culture using a filter press, thus obtaining a γ-PGA-containing sample solution.
[0030]2N sulfuric acid solution was added to the γ-PGA-containing sample solution and left to stand at 10° C. for 12 hours to collect a γ-PGA precipitate. The collected precipitate was washed with a sufficient amount of distilled water to obtain γ-PGA using a Nutsche filter. The obtained γ-PGA was measured for molecular weigh...
example 2
Toxicity Test Results Upon Oral Administration of γ-PGA
[0031]In order to examine the safety upon oral administration of γ-PGA, toxicity test upon a single oral administration of poly-gamma-glutamic acid using rats was performed by Biotoxtech Co., Ltd., an institute approved by GLP (Good Laboratory Practice) in accordance with Biotoxtech Standard Operating Procedures (SOPs), Good Laboratory Practice (GLP) regulations and test guideline.
[0032]Ten, 6-week-old male rats (159.76˜199.27 g) and 10 female rats (121.60˜138.80 g) were used, and the dose of γ-PGA administered to individual rats was calculated on the basis of body weight measured on the day of administration after fasting. All rats were fasted for about 16 hours but had free access to drinking water before administration, and then they were subjected to forceful oral administration with a single dose of γ-PGA by stomach tube using a disposable syringe (5 ml) having a catheter for oral administration attached thereto, followed b...
example 3
Immune Enhancement Effect 1 of γ-PGA Against Influenza Virus
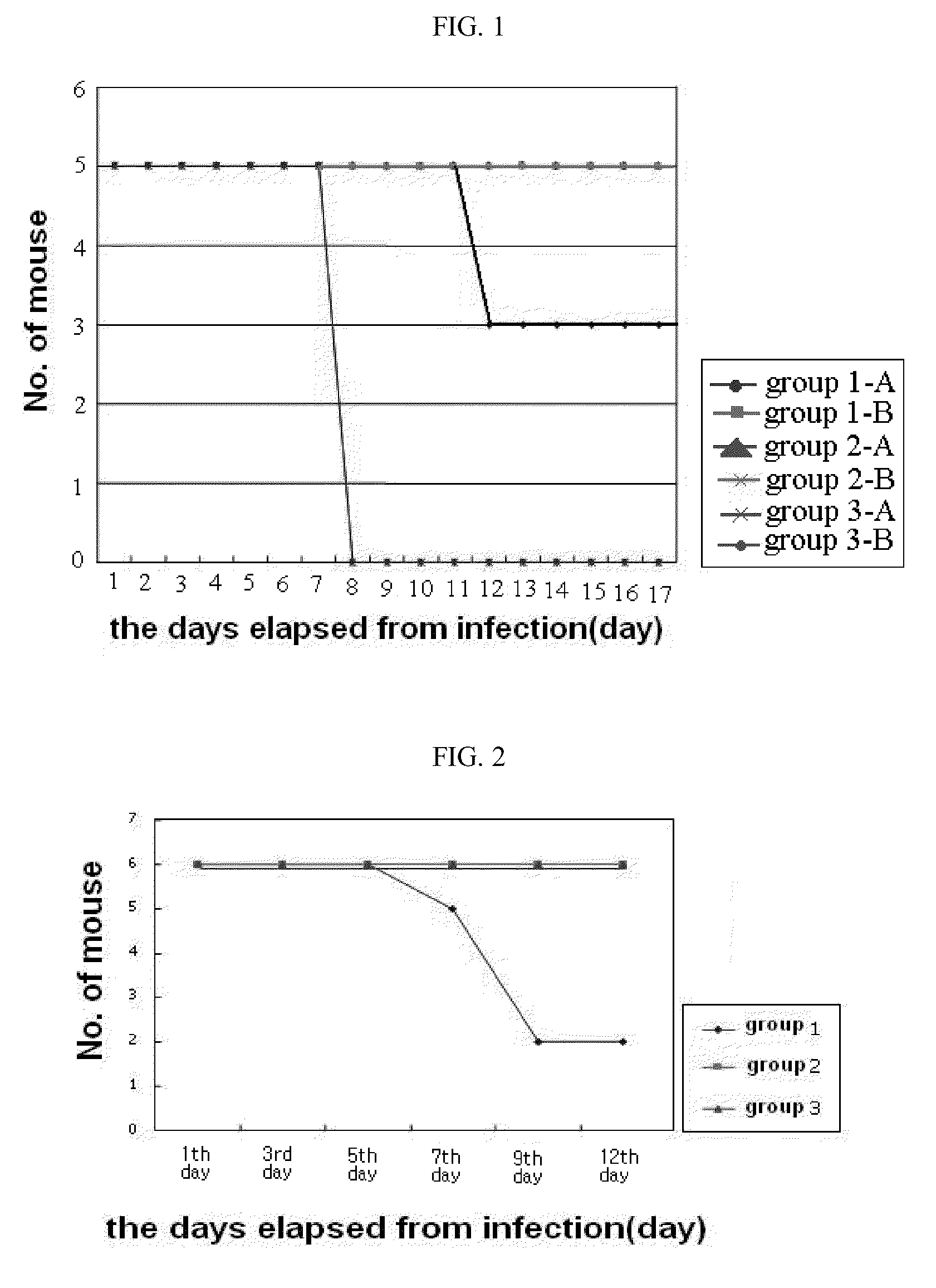
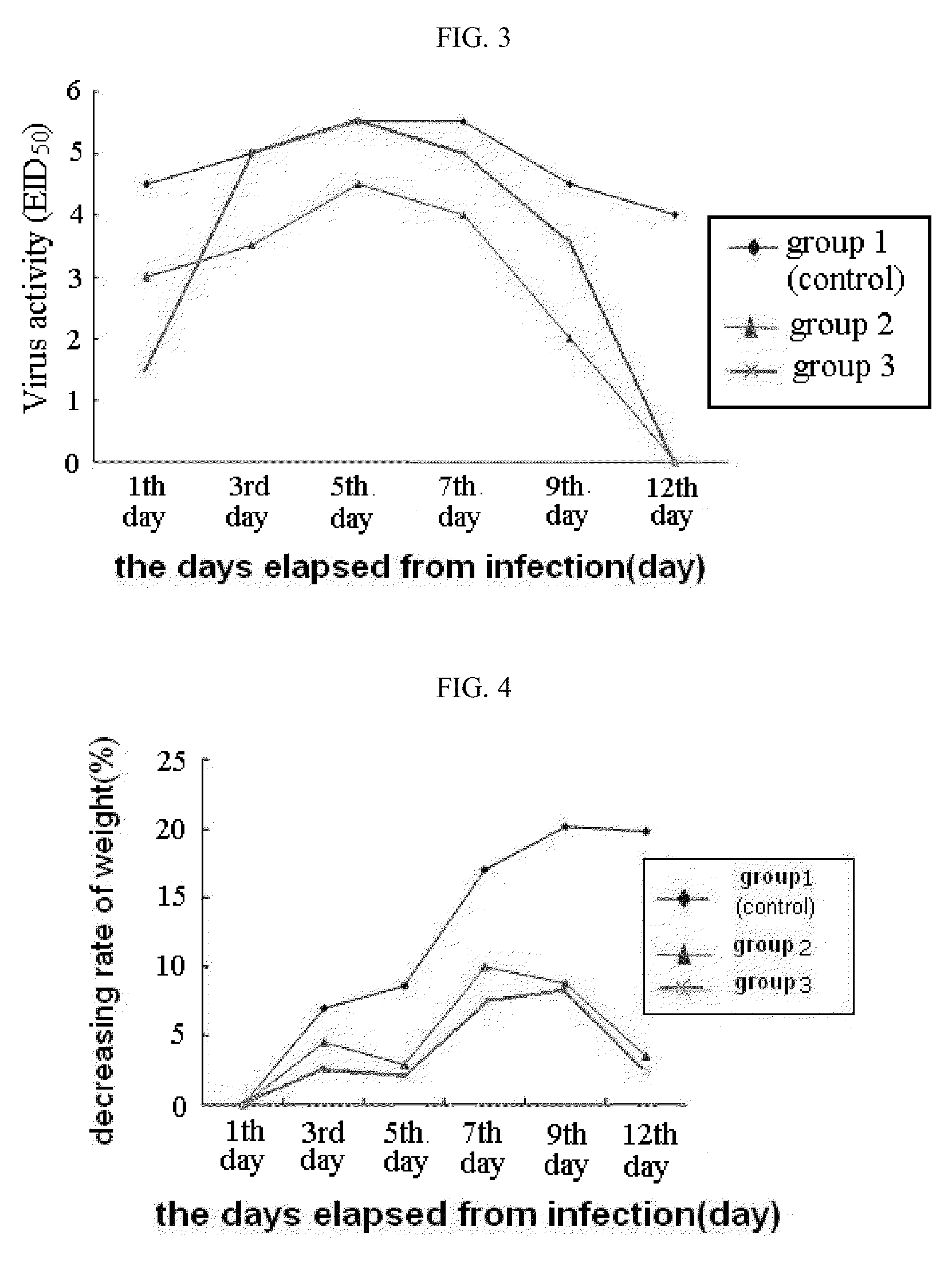
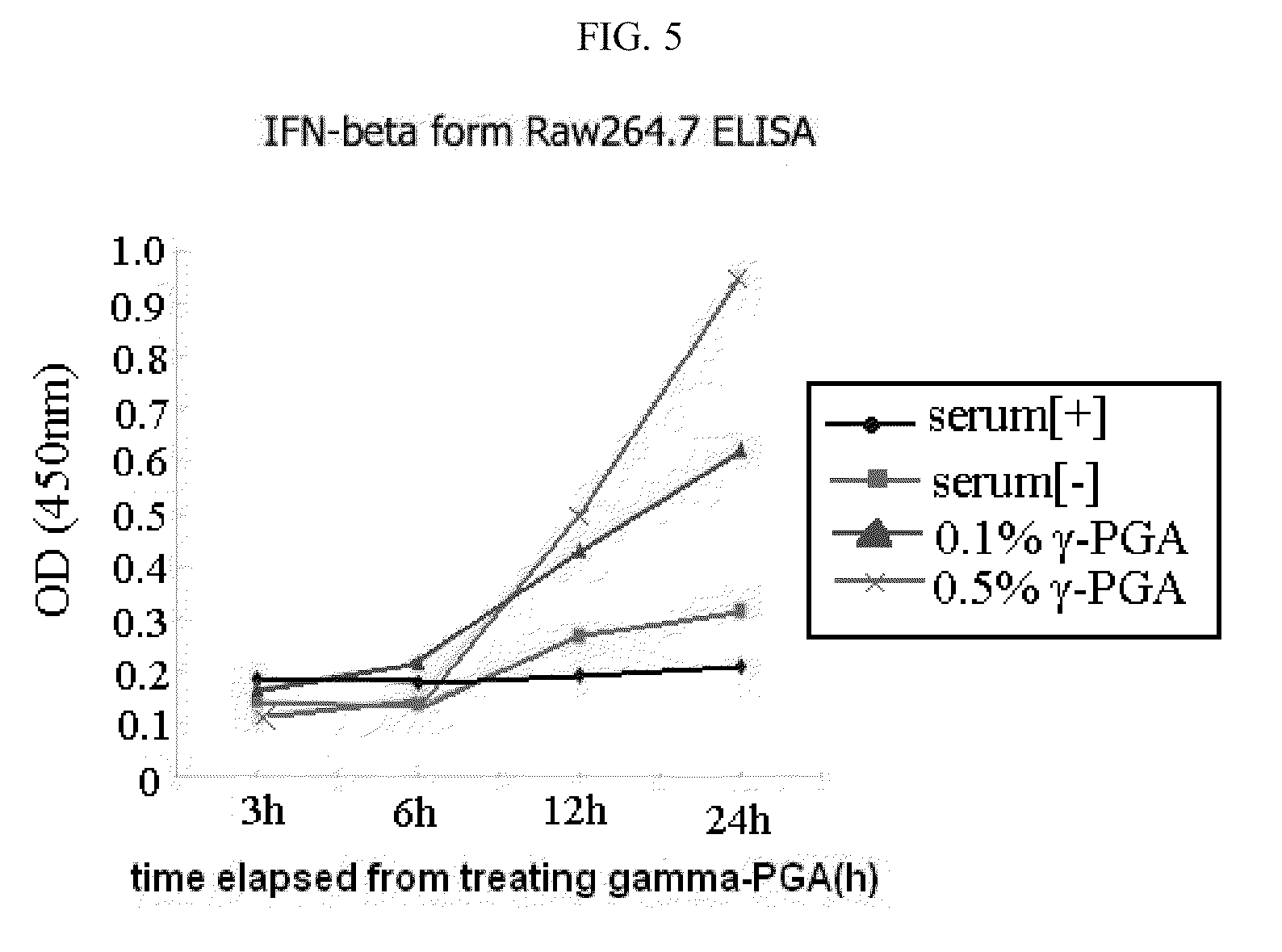
[0035]In the present example, in order to examine an infection-inhibiting effect of poly-gamma-glutamic acid specific to avian influenza virus, the animal's death, virus proliferation, and antibody production in experimental animals infected with influenza virus, were analyzed.
(1) Preparation of Virus
[0036]As influenza virus used as a pathogen, H1N1 high pathogenicity influenza virus strain (A / Puerto Rico / 8 / 34 (H1N1)) was isolated from a mouse which was donated by Prof. Choi, Young-Ki of microbiological laboratory, College of Medicine, Chungbuk National University to amplify in Madin-Darby canine kidney (MDCK) cells to use, and 6-week-old female Balb / C mice were used as experimental animals.
[0037]Isolation of pure virus was carried out as follows.
[0038]Firstly, the isolated virus was diluted in antibiotic-containing PBS to inoculate into 10-day-old embryonated white laying hen's eggs, and then subjected to a stationary cult...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Atomic weight | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com