Application of Verteporfin in preparation of medicine for resisting novel coronavirus SARS-CoV-2
A technology of verteporfin and coronavirus, applied in the field of medicine, can solve the problem that verteporfin has not been seen to inhibit coronavirus, etc., and achieve the effect of inhibiting infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
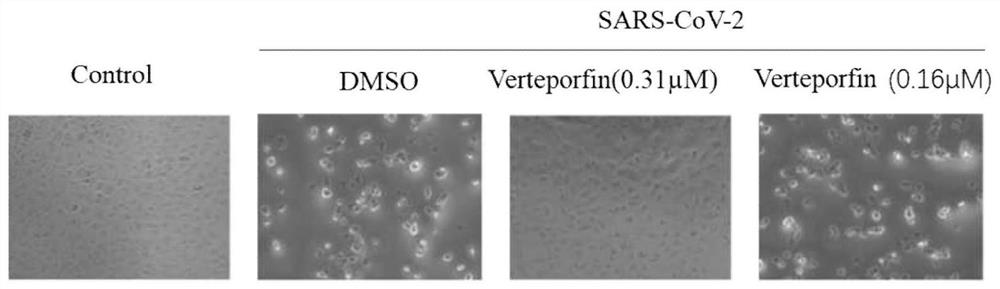
[0027] The inhibitory effect of different concentrations of verteporfin on the infection of SARS-CoV-2 on Vero-E6 cells was observed by cytopathic (CPE):
[0028] Inoculate Vero-E6 cell suspension (4 × 10 4 cells / well), the culture plate was placed in the incubator for pre-cultivation for 12 hours, and the cells adhered to the wall for growth;
[0029] Add the corresponding concentration of verteporfin to pre-treat for 1 hour, add DMSO to the solvent control, and add no drug to the negative control;
[0030] Then add 200PFU SARS-CoV-2 virus (GenBank: MT121215.1) to each well except the negative control. After 12 hours of infection, wash twice with PBS, add new medium containing verteporfin, and incubate at 37°C After 48 hours, the cytopathic changes were observed under the microscope.
[0031] Experimental results such as figure 1 As shown, the experimental results show that normal Vero-E6 cells (negative control) grow normally after 48 hours of culture, and no cytopathic c...
Embodiment 2
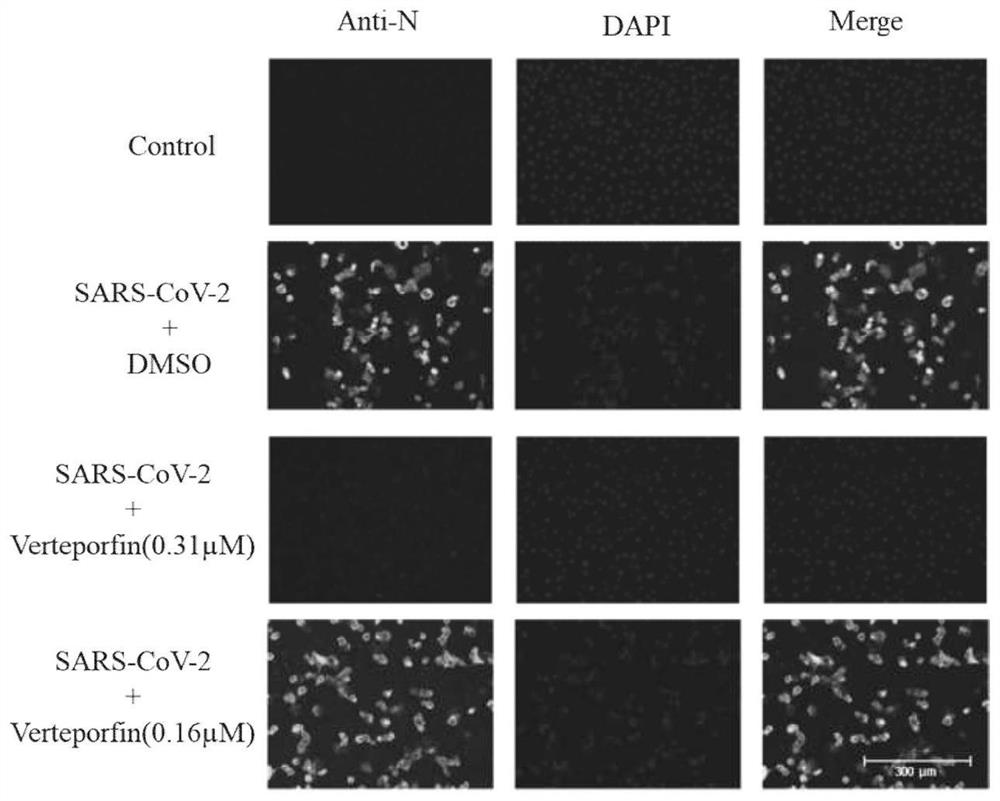
[0033] The inhibitory effect of different concentrations of verteporfin on the infection of SARS-CoV-2 on Vero-E6 cells was observed by indirect immunofluorescence experiment:
[0034] Cells were processed as in Example 1, and washed 2 times with PBS after virus infection for 48 hours, each time for 5 minutes;
[0035] Add 4% paraformaldehyde to fix at room temperature for 15 minutes;
[0036] Discard the solution, add PBS solution containing 0.5% Triton X-100, react at room temperature for 10 minutes, wash with PBS twice, 5 minutes each time;
[0037] Add freshly prepared PBS solution containing 5% BSA, block at room temperature for 1 hour, wash with PBS twice, 5 minutes each time;
[0038] Add 1:1000 diluted mouse anti-N protein (SARS-CoV-2) polyclonal antibody, and react at room temperature for 1 hour;
[0039] Wash with PBS 3 times, 5 minutes each time;
[0040] Add FITC-goat anti-mouse IgG secondary antibody diluted 1:10000, react at room temperature for 1 hour; wash w...
Embodiment 3
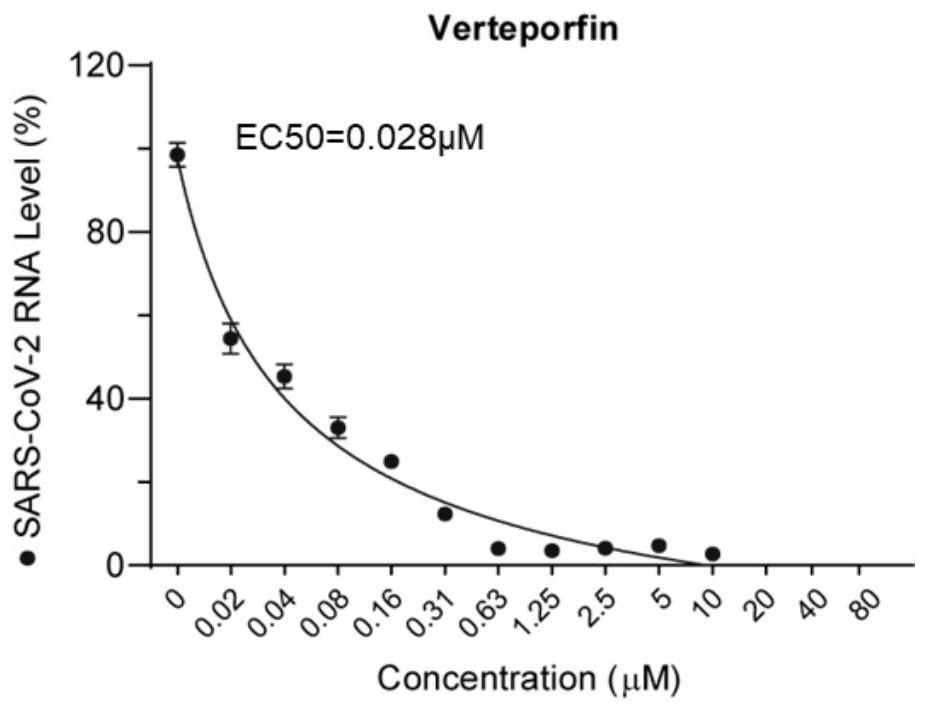
[0045] Real-time quantitative PCR (Q-RT-PCR) method was used to detect the viral copy number of Vero-E6 cell supernatant to verify the inhibitory effect of verteporfin on the infection of SARS-CoV-2:
[0046] Cells are processed as in example 1 step;
[0047] After 48 hours, the cell supernatant was collected for RNA extraction.
[0048] RNA extraction:
[0049] Add 100 μL of cell supernatant to 300 μL TRIzol lysate, mix well and lyse;
[0050] Add 200 μL of chloroform and shake vigorously, let stand for 2-3 minutes, then centrifuge at 12000g for 15 minutes at 4°C;
[0051] Aspirate the supernatant, add 500 μL isopropanol, let stand for 10 minutes, and then centrifuge at 12000g for 10 minutes at 4°C;
[0052] Add 1 mL of 75% ethanol to wash the RNA pellet; centrifuge at 7500g for 5 minutes at 4°C;
[0053] Discard 75% ethanol, add 20-50 μL water to dissolve the RNA precipitate after drying;
[0054] RNA reverse transcription:
[0055] For reverse transcription of RNA, cD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com