Ros-responsive microneedle patch for acne vulgaris treatment
A responsive, therapeutic technology for the preparation of microneedle arrays, compositions comprising absorbent materials, microneedle arrays and skin patches, for the treatment of acne or other inflammatory/infectious skin diseases, in the field of microneedles, capable of Solve the problems of intestinal flora damage, adverse side effects, teratogenic effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
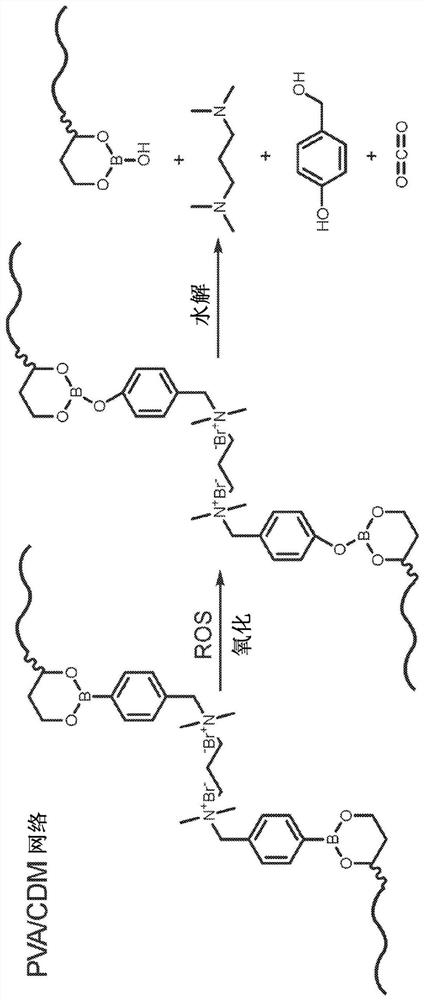
[0198] Preparation of ROS-responsive hydrogels
[0199] All chemicals were purchased from Sigma-Aldrich (St. Lious, Missouri, United States of America) and used as received unless otherwise stated. Purification system by Millipore NanoPure (MilliporeSigma, Burlington, Massachusetts, United States of America, resistivity higher than 18.2MΩcm -1 ) to prepare deionized water.
[0200] Synthesis of ROS-responsive crosslinker (TSPBA): 4-(bromomethyl)phenylboronic acid (1 g, 4.6 mmol) and N,N,N',N'-tetramethyl-1,3-propanediamine (0.2 g, 1.5 mmol) was dissolved in dimethylformamide (DMF; 40 mL), and the solution was stirred at 60° C. for 24 h. Afterwards, the mixture was poured into tetrahydrofuran (THF, 100 mL), filtered, and washed with THF (3 x 20 mL). After drying under vacuum overnight, pure TSPBA was obtained (0.6 g, 70% yield). 1 H-NMR (300MHz, D 2 O, δ): 7.677 (d, 4H), 7.395 (d, 4H), 4.409 (s, 4H), 3.232 (t, 4H), 2.936 (s, 6H), 2.81 (m, 2H). The synthetic route and struct...
Embodiment 2
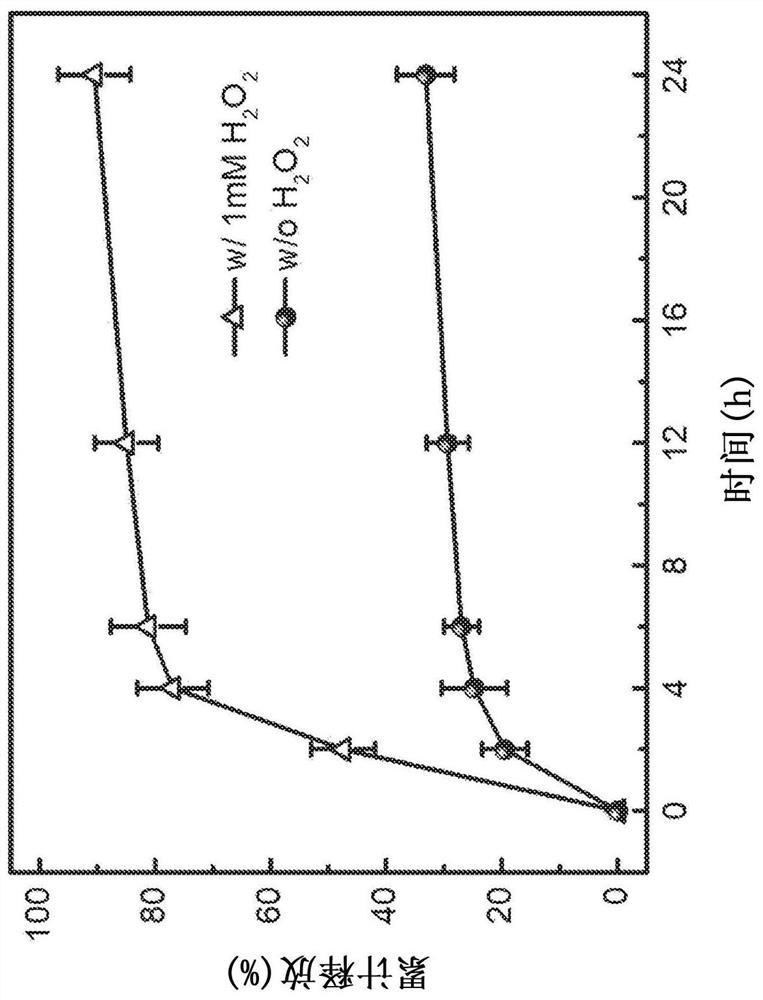
[0206] In vitro results of ROS-responsive hydrogels
[0207] In vitro release profile: by dissolving the hydrogel in 12mL PBS buffer (NaCl, 137mM; KCl, 2.7mM; Na 2 HPO 4 , 10mM; KH 2 PO 4 , 2mM, pH 7.4), incubated on an orbital shaker at 37°C, to which was added H 2 o 2 Up to a concentration of 1 mM, the in vitro release profile of CDM released from RR-PVA gel was evaluated. At predetermined time points, the concentration of CDM in the supernatant was determined by high performance liquid chromatography (HPLC). On the Zorbax Eclipse Plus RRHD C18 column (2.1 × 50mm, 1.8μm particle size) (Agilent Technologies, Santa Clara, California, United States of America) using 80% water (HPLC grade, containing 0.1% v / v trifluoroacetic acid ) and 20% acetonitrile (HPLC grade, containing 0.1% v / v trifluoroacetic acid) was used to analyze samples at 205 nanometers (nm). Cumulative release (%) is expressed as a percentage of total drug released over time.
[0208] Antibacterial effect...
Embodiment 3
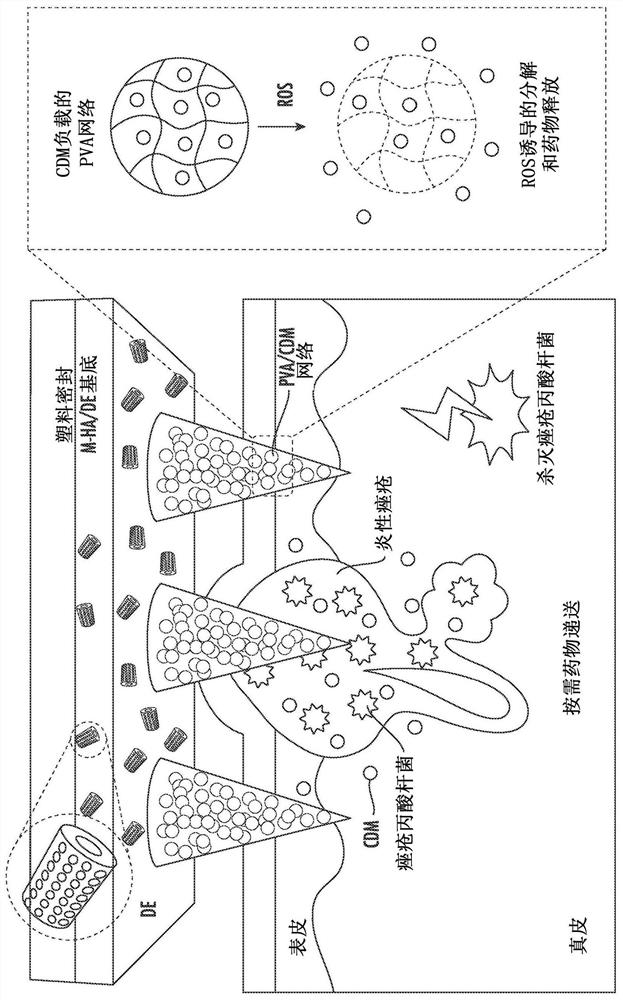
[0213] Preparation of ROS-responsive microneedle patch
[0214] Preparation of ROS-responsive (RR) microneedle patches: All MN patches in this study were prepared using homogeneous silicone molds from Blueacre Technology Ltd (Dundalk, Ireland). There is an array of 11 x 11 needles in the mold, each needle has a height of 600 μm and a circular base diameter of 300 μm. The tip-to-tip spacing is 600 μm. First, PVAMNs were formed by pipetting a premixed PVA (3 wt%) / TSPBA (3 wt%) (ratio 3:1) solution (400 μL) onto the MN mold surface. The mold was kept under vacuum (600mmHg) for 30 minutes to allow the solution to deposit into the MN cavity. Afterwards, the covered mold was centrifuged at 500 rpm for 20 minutes using a Hettich Universal 32R centrifuge (Hettich GmbH & Co. KG, Tuttlingen, Germany) to concentrate the PVA network in the MN. In the first step, drug-loaded MNs were fabricated by adding a predetermined amount of drug in the PVA / TSPBA solution. Non-responsive (NR) micr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com