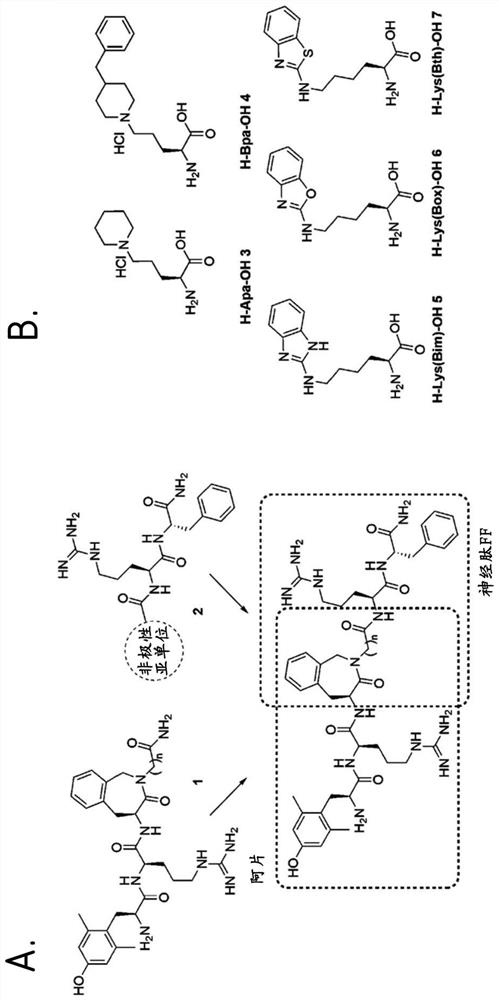
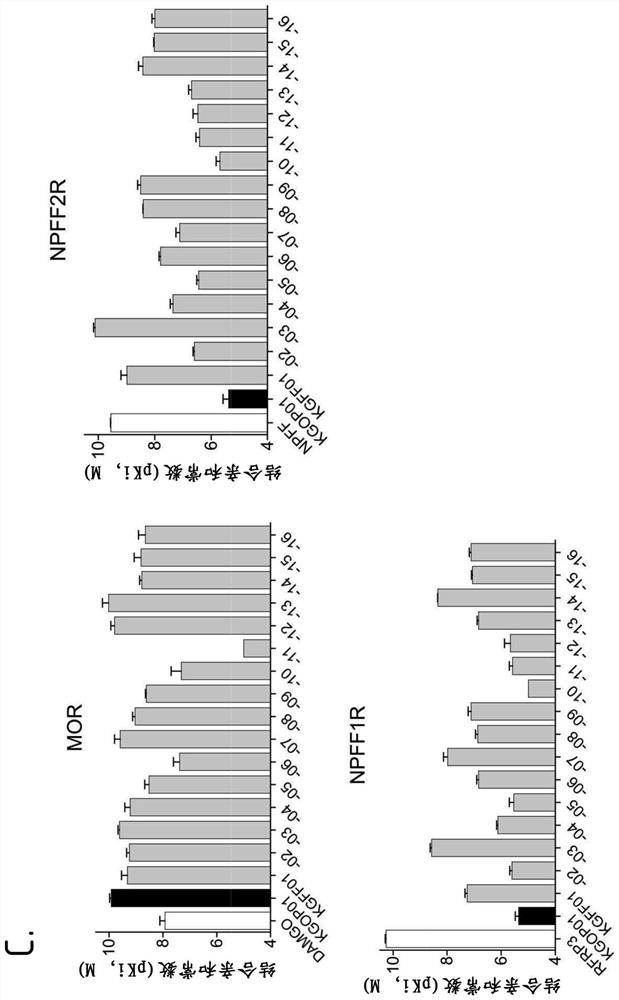
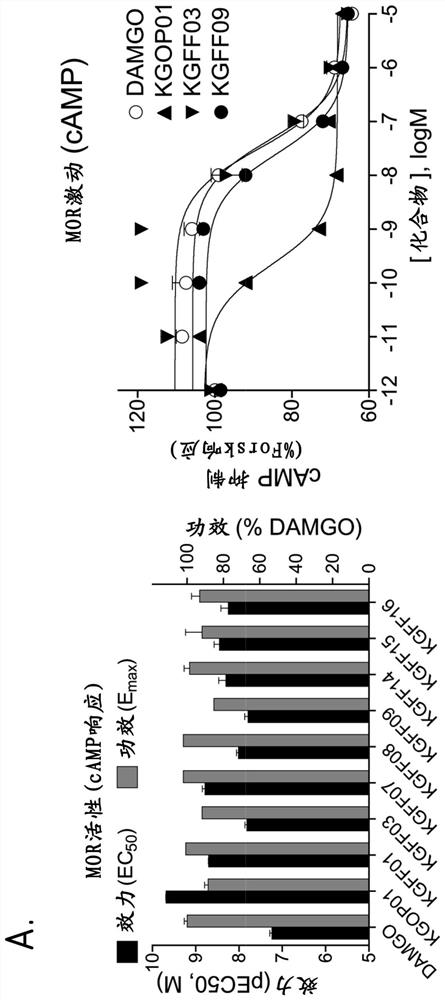
Hybrid mu opioid receptor and neuropeptide ff receptor binding molecules, their methods of preparation and applications in therapeutic treatment
A molecular, alkyl-based technology applied in the field of hybrid μ-opioid receptor and neuropeptide FF receptor-binding molecules, their preparation and use in therapy, capable of solving little-studied problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0376]1. Materials and methods
[0377]1.1. Chemical synthesis
[0378]1.1.1. Peptide KGFF01-KGFF07
[0379]The peptides KGFF01-KGFF07 were manually synthesized by standard Fmoc-SPPS on Rink amide AM resin. Standard coupling was carried out for 1.5 h by using 3 equivalents (equiv.) of Fmoc protected amino acids and 3 equivalents of coupling reagent (HCTU) in 0.4NMM DMF. Fmoc-Aba-β-Ala-OH was only coupled with a 1.5 equivalent excess of Fmoc-dipeptide and the coupling agent for 3 hours. Boc-Dmt-OH was coupled by using 1.5 equivalents of amino acids in DMF and 1.5 equivalents of HOBt / DIC for 2h without adding alkali to avoid coupling with unprotected phenolic groups. For Fmoc-deprotection, the resin was treated with 20% 4-methylpiperidine in DMF for 5 and 15 minutes, or with DBU / piperidine / DMF 2 / 2 / 96 for 3×30s and 7 minutes. After each coupling and after the deprotection step, use DMF (3×), iPrOH or MeOH (3×) and CH2Cl2(3×) Wash the resin. Use the cutting mixture (TFA / TES / H2O 95:2.5:2.5, TES c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com