New application of acevaltratum
A technology of acetyl valerian and combined drug, applied in the field of pharmacology, can solve problems such as multiple myeloma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
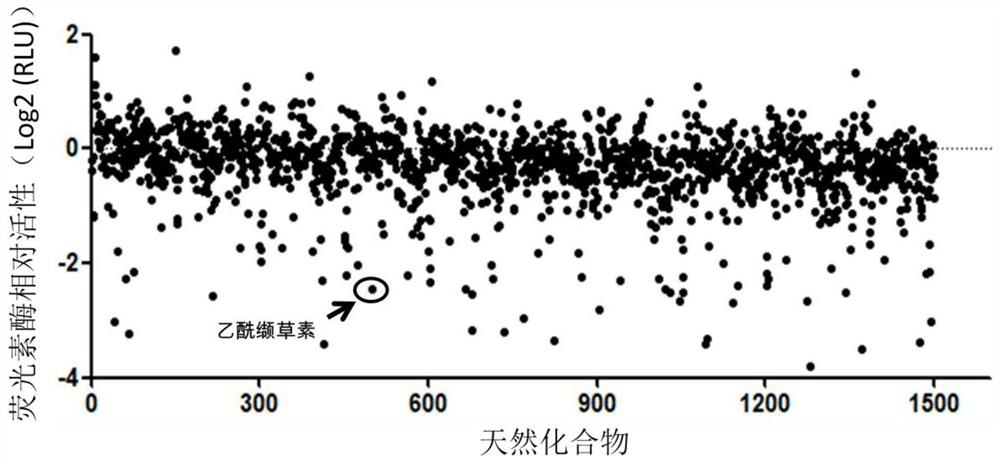
[0048] Transfect Otub1, HA-c-Maf, and c-Maf-specific recognition unit (pMARE)-driven luciferase.Luci.Plasmid pMARE.Luci in HEK293T cells, and cells were cultured for 24 hours at 10,000 cells per well Inoculate into 96-well cell culture plate, add different natural compounds (all compounds are dissolved in dimethyl sulfoxide (DMSO) and then diluted to the application concentration after the cells adhere to the wall. After continuing to culture at 37°C for 24 hours, collect the cells for fluorescein Determination of enzyme activity. The relative luciferase activity (RLU) of each compound treated cells is indicated by the Log2 value of the luciferase in the treated sample divided by the luciferase in the DMSO control cell.
[0049] See results figure 1 ,according to figure 1 , acetylvalerianin significantly inhibited c-Maf-driven luciferase activity.
Embodiment 2
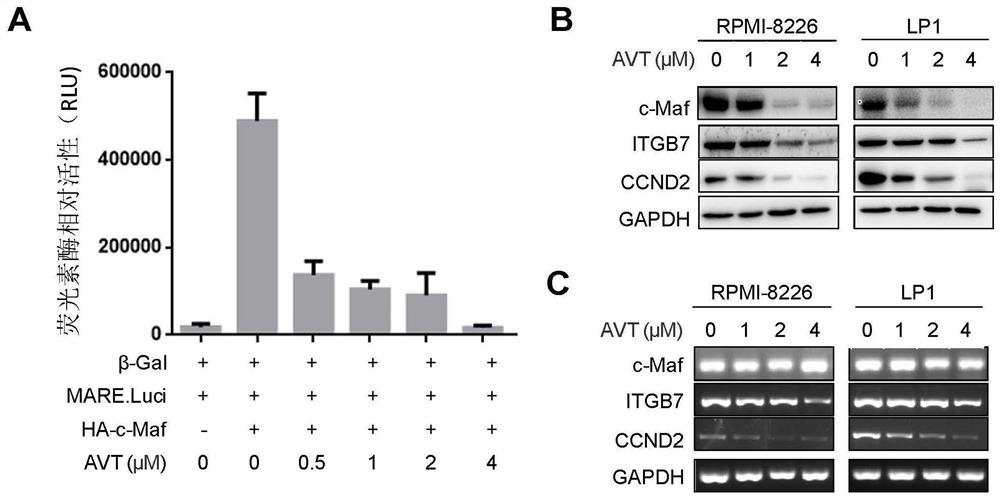
[0051] In order to further analyze the inhibitory effect of acetylvalerian (AVT) on the oncogene transcription factor c-Maf, the present invention first transfected Otub1, HA-c-Maf, pMARE.Luci. plasmid and internal control β-Gal in HEK293T cells . After 24 hours, the cells were inoculated into 96-well cell culture plates at a density of 10,000 cells per well. After the cells adhered to the wall, different concentrations of acetylvalerianin were added, and the cells were collected for luciferase activity assay after continuing to culture at 37°C for 24 hours. , to analyze the activity of acetylvalerianin in inhibiting luciferase.
[0052] Further, in the present invention, the multiple myeloma cell lines (RPMI-8226, LP1) are treated with different concentrations of acetylvalerian (0 μM, 1 μM, 2 μM, 4 μM, and the medicine is prepared with 100% DMSO and then diluted in serum-free IMDM. After 24 hours of treatment in the base, the final concentration of DMSO was less than 0.1%, t...
Embodiment 3
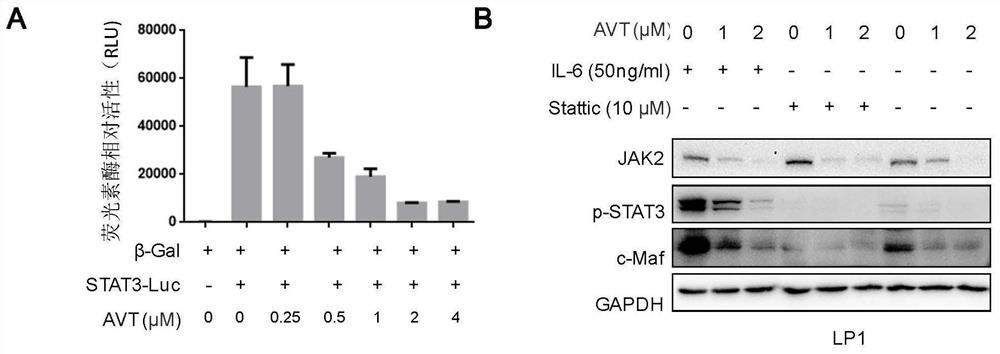
[0056] In order to analyze the inhibitory effect of acetylvalerian (AVT) on the oncogene transcription factor STAT3, the present invention transfected the luciferase plasmid (pSTAT3-Luc) driven by the STAT3 specific recognition unit and the internal control β-Gal plasmid in HEK293T cells . After 24 hours, the cells were inoculated into a 96-well cell culture plate at a density of 10,000 cells per well, and different concentrations of acetylvalerian were added after the cells adhered to the wall. The culture was continued at 37°C for 24 hours, and then the cells were collected for the determination of luciferase activity. Acetylvalerianin inhibited the activity of luciferase. In addition, after the multiple myeloma cell line LP1 was treated with different concentrations of acetylvalerianin (0, 1, 2, 4 μM) or co-treated with interleukin 6 (IL-6) or STAT3 inhibitor Stattic for 24 hours, the total cell protein was extracted , JAK2, p-STAT3 and c-Maf protein levels were detected b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com