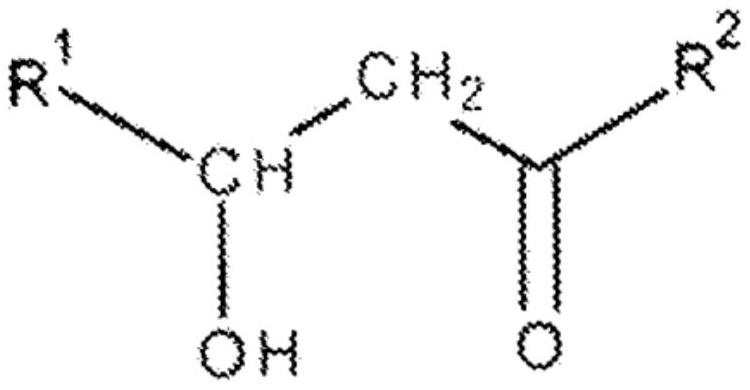
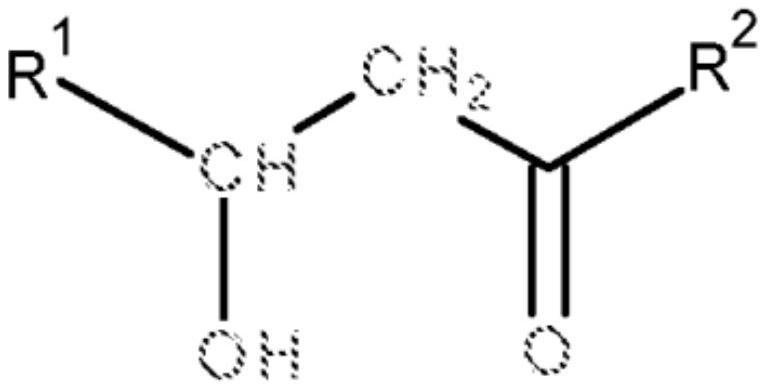
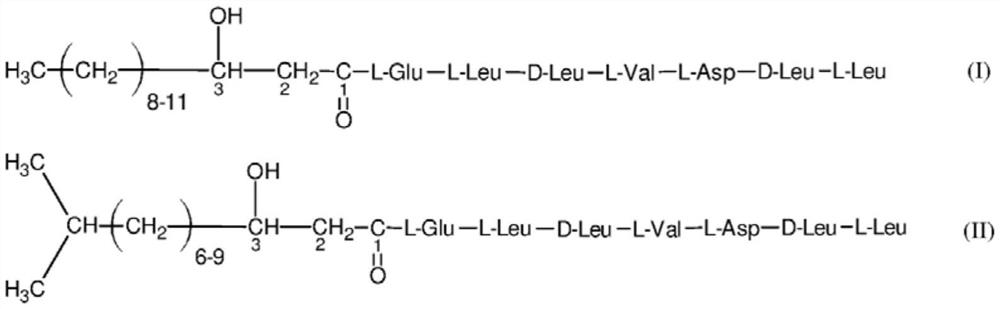Method of removing lipopeptides from solutions and changing their structure
A technology of lipopeptide and solution, applied in the field of changing the structure of lipopeptide, can solve the problems of no environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Surfactin is released from a post-fermentation solution containing a mixture of lipopeptides with a predominant surfactin content using known methods. 100ml of the resulting 0.8mg / ml surfactin aqueous solution was placed in a 250ml Erlenmeyer flask. Then add 1 gram of activated carbon, the particle size of activated carbon is 0.2 to 1mm, and the pore area is 650m 2 / g, and the pH of its aqueous suspension is 8.5. The suspension was mixed using a shaker at 20°C for 72 hours. After 72 hours, the solution was separated from the charcoal by filtration and centrifugation. The amount of adsorbed surfactin retained on the activated carbon was 5% of that originally introduced into the solution. The remainder of surfactin reacts on the surface of the activated carbon to form surfactin hydrolyzate having a linear structure represented by formulas (I) and (II).
[0025]
[0026] Figure 1. The amino acid sequence is the formula of the linear analogue of surfactin of L-Glu-L-Le...
Embodiment 2
[0028] Same as Example 1, the only difference is that the concentration of surfactin aqueous solution is 2 mg / ml. Subsequently, 3 g of activated carbon was introduced with a particle size of 0.1 to 0.2 mm and an internal surface area of 750 m 2 / g, pH 9 in aqueous suspension. The suspension was mixed using a shaker at 25°C for 72 hours. After 72 hours, the solution was separated from the charcoal by filtration and centrifugation. Surfactin was adsorbed on activated carbon in an amount of 3% initially introduced into the solution. The remainder of the surfactin reacts on the carbon surface to form a linear structured surfactin hydrolyzate. The mixture of linear products was separated into analogs with different alkyl chain lengths by preparative liquid chromatography, the structure of which is shown in formula (III).
[0029]
[0030] R 1 - Alkyl chains with 9 to 12 carbon atoms
[0031] Fig. 2 amino acid sequence is the formula of the linear analogue of surfactin of...
Embodiment 3
[0033] Same as Example 1, except that the surfactin solution was applied to the column. In aqueous suspension, the column is filled with 20 grams of activated carbon in the form of a monolith with a mesh size of 0.5 x 0.5 mm, a wall thickness of 0.5 mm and a pore area of 750 m 2 / g, pH value is 9. At 25° C., 2000 ml / h of 2 mg / ml surfactin in water was introduced from above using a peristaltic pump. After passing through the activated carbon bed, the solution is directed to a treatment tank from which it is drawn using a pump for treatment in a flow system. This treatment was carried out for 48 hours. Surfactin was adsorbed on activated carbon in an amount of 10% initially introduced into the solution. The remaining part of surfactin reacted on the surface of activated carbon to form a linear structure of surfactin hydrolyzate, as shown in formulas (IV)–(VI), which remained in the circulating solution. Charcoal can be used multiple times in this process. Charcoal can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com