Alkylamine substituted cantharidin derivative, preparation method, pharmaceutical composition and application thereof
A cantharidin derivative, the technology of cantharidin, which is applied in the field of medicine and can solve the problems of restricted use, restricted toxicity, and prevented cancer treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
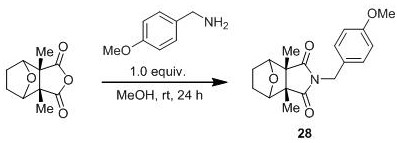
[0034] Preparation of Compound 11
[0035]
[0036] Add cantharidic anhydride (39.2 mg, 0.2 mmol) and methanol (2.0 mL) sequentially into a 10-mL pressure-resistant reaction tube equipped with a stirring bar, seal with a rubber stopper, cool to 0 °C, and add N,N-di Methylethylenediamine (18.0 mg, 0.2 mmol) in methanol (1.0 mL) was added dropwise, stirred for 2-8 minutes, removed from the ice bath, and slowly raised to room temperature for another 24 hours. After the reaction was completed, the mixture was directly concentrated under reduced pressure, and the crude product was separated by column chromatography to obtain white solid 11 (25.0-50.0mg, 47-94% yield). 1 H NMR (300 MHz, CDCl 3 ) δ 4.52 (s, 2H), 3.56 (t, J = 7.1 Hz, 2H), 2.41 (t, J = 7.0 Hz, 2H), 2.21 (s, 6H), 1.80 – 1.63 (m, 4H), 1.10 (s, 6H) ppm; MS (ESI) m / z Calcd for [C 14 h 22 N 2 o 3 +Na] + 267.3, found 267.1; MS(ESI) m / z Calcd for [C 14 h 22 N 2 o3 +H] + 267.1703, found 267.1707. ...
Embodiment 2
[0065] According to the method of Example 1, cantharidin derivatives were first prepared, and water for injection was added as usual, finely filtered, potted and sterilized to make an injection.
Embodiment 3
[0067] According to the method of Example 1, cantharidin derivatives were first prepared, dissolved in sterile water for injection, stirred to dissolve, filtered with a sterile suction filter funnel, then sterile fine filtered, subpackaged in ampoules, and freeze-dried at low temperature Afterwards, it is sterilized and melt-sealed to obtain a powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com