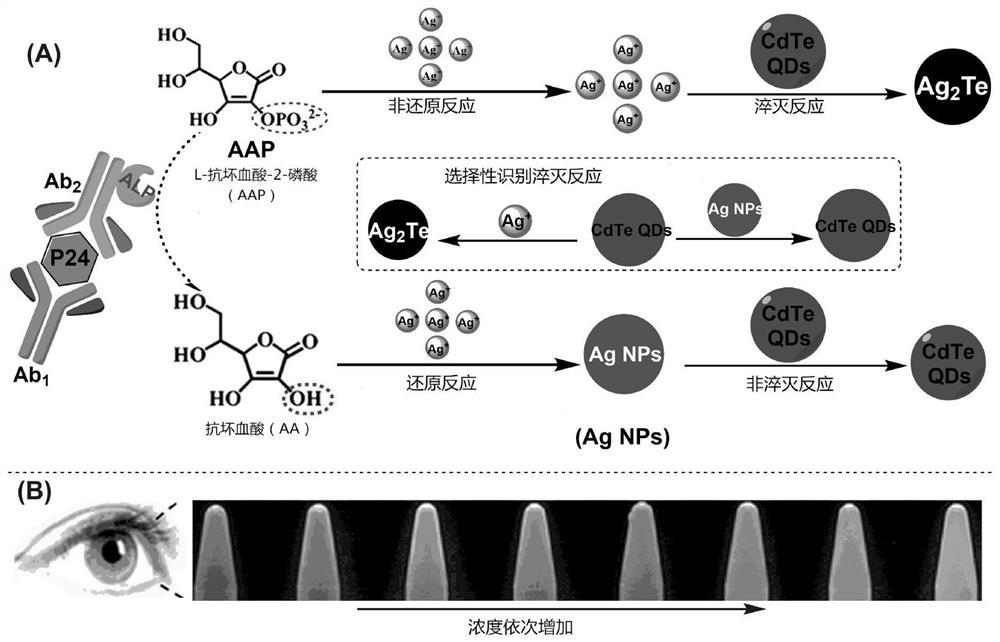
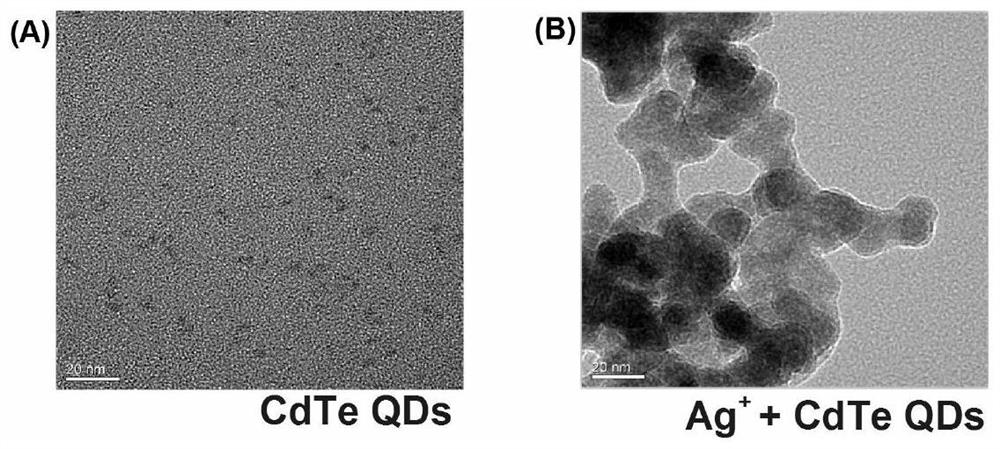
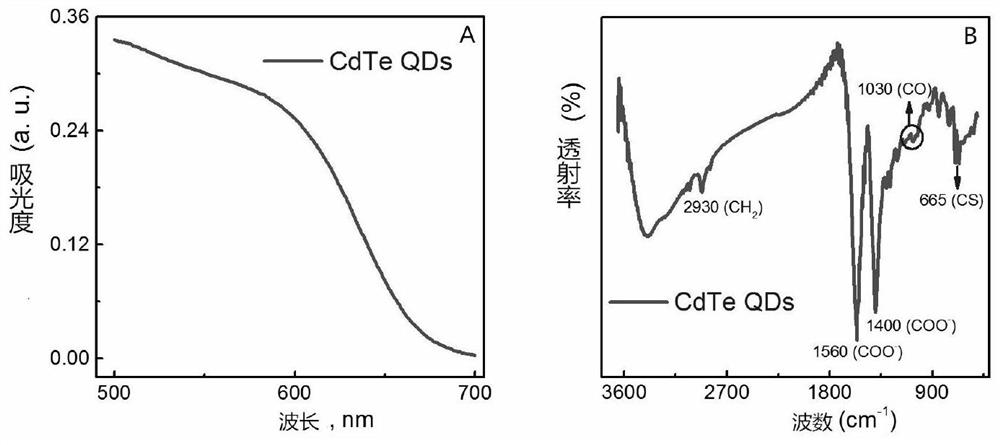
HIV p24 high-sensitivity detection method based on quantum dot selective cation exchange reaction
A technology of cation exchange and detection method, which is applied in the direction of immunoassay, measuring device, instrument, etc., can solve the problems of high cost, low analytical sensitivity of ultraviolet absorption spectrometer, difficulty in realizing portable visual rapid analysis, etc., achieve high long-term stability, improve Effects of analytical sensitivity and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052](1) Add 10μL of 20μg / mL recognition antibody (Ab1) Was mixed with 30μL of analysis buffer and added to a 96-well plate, and incubated overnight at 4°C. Wash the 96-well plate 3 times with 200 μL of washing buffer to remove unbound recognition antibody. Subsequently, 100 μL of blocking buffer was added and incubated at 37°C for 1 hour to seal the remaining vacancies in the 96-well plate, and washed 3 times. Subsequently, 40 μL of the serum sample to be tested and 60 μL of analysis buffer were added to the 96-well plate, incubated at 37° C. for 1 hour, and washed three times. 40μL of 10μg / mL biotin-labeled detection antibody (Ab2) Was added to a 96-well plate together with 60 μL of assay buffer, and incubated at 37°C for 1 hour to form a primary antibody-p24-detection antibody-biotin sandwich complex, and washed three times.
[0053](2) Add 40 μL of 5 μg / mL streptavidin-alkaline phosphatase (SA-ALP) and 60 μL of analysis buffer to the 96-well plate obtained in step (1), incubate at...
Embodiment 2
[0057](1) Add 10μL of 30μg / mL recognition antibody (Ab1) Was mixed with 10μL of analysis buffer and added to a 96-well plate, and incubated overnight at 2°C. Wash the 96-well plate twice with 200 μL of washing buffer to remove unbound recognition antibody. Subsequently, 100 μL of blocking buffer was added and incubated at 37° C. for 1 hour to seal the remaining vacancies in the 96-well plate, and washed twice. Subsequently, 40 μL of the serum sample to be tested and 40 μL of analysis buffer were added to the 96-well plate, incubated at 35° C. for 80 min, and washed three times. 40μL of 5μg / mL biotin-labeled detection antibody (Ab2) Was added to a 96-well plate together with 40 μL of assay buffer, and incubated at 35°C for 80 min to form a primary antibody-p24-detection antibody-biotin sandwich complex, and washed twice.
[0058](2) Add 40 μL of 2 μg / mL streptavidin-alkaline phosphatase (SA-ALP) and 40 μL of assay buffer to the 96-well plate obtained in step (1), incubate at 20°C for 20...
Embodiment 3
[0061](1) Add 10μL of 10μg / mL recognition antibody (Ab1) Was mixed with 50μL of analysis buffer and added to a 96-well plate, and incubated overnight at 6°C. Wash the 96-well plate 4 times with 200 μL of washing buffer to remove unbound recognition antibody. Subsequently, 100 μL of blocking buffer was added and incubated at 37° C. for 1 hour to seal the remaining vacancies in the 96-well plate, and washed 4 times. Subsequently, 40 μL of the serum sample to be tested and 80 μL of analysis buffer were added to the 96-well plate, incubated at 39° C. for 40 min, and washed three times. 40μL of 15μg / mL biotin-labeled detection antibody (Ab2) Add 80μL of assay buffer to a 96-well plate and incubate at 39°C for 40min to form a primary antibody-p24-detection antibody-biotin sandwich complex, and wash 4 times.
[0062](2) Add 40 μL of 8 μg / mL streptavidin-alkaline phosphatase (SA-ALP) and 80 μL of analysis buffer to the 96-well plate obtained in step (1), incubate at 30°C for 10 minutes and was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com