ASK1 inhibitor, derivative, preparation method, pharmaceutical composition and application thereof
A technology of derivatives and inhibitors, applied in the field of ASK1 inhibitors and its derivatives, can solve the problems of non-alcoholic steatohepatitis not reaching the clinical endpoint and single structure type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
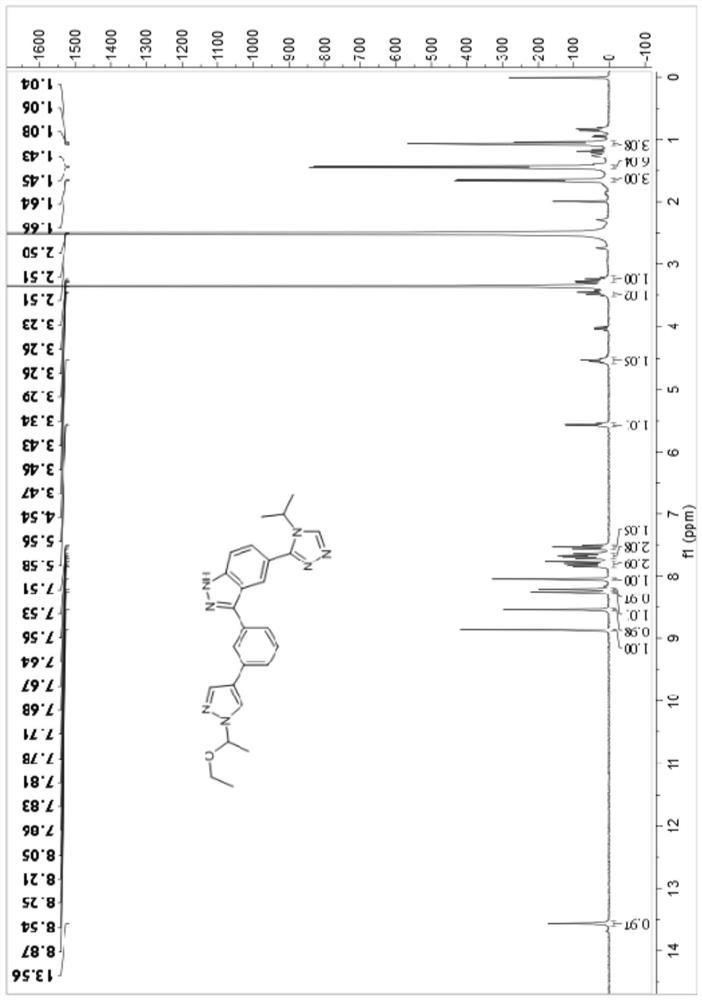
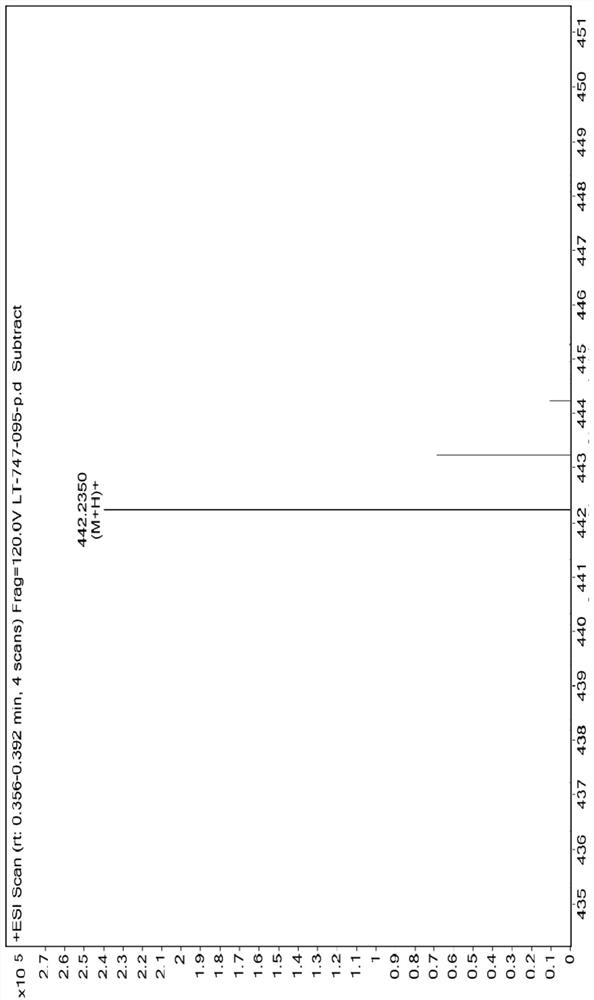
[0116] Example 1: 3-(3-(4-cyclopropyl-1H-imidazol-1-yl)phenyl)-5-(4-isopropyl-4H-1,2,4-triazole-3- base)-1H-indazole (compound I-1) synthesis
[0117]
[0118] 1. 4-cyclopropyl-1-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1H- Synthesis of Imidazole (Compound 1-1)
[0119]
[0120] (1) Synthesis of 2-((3 bromophenyl)amino)-1-cyclopropyl-1-ethanone (compound 3)
[0121] m-Bromoaniline (3.0g, 17.5mmol) was dissolved in DMF, added K 2 CO 3 (2.9g, 21mmol), KI (2.9g, 17.5mmol), stirred at room temperature for 30min, added 2-bromo-1-cyclopropylethan-1-one (4.25g, 26.2mmol) and reacted at 60°C for 3h. The end of the reaction was detected by TLC, the reaction solution was diluted with water, and then extracted with EA, which was dried and spin-dried, and the target product (3 g, 68%) was obtained by silica gel column chromatography. ESI-MS m / z:254.0[M+H] + .
[0122] (2) Synthesis of 1-(3-bromophenyl)-4-cyclopropyl-1H-imidazole-2-thiol (compound 4)
[0123] 2...
Embodiment 2
[0144] Example 2: N-cyclopropyl-3-(5-(4-isopropyl-4H-1,2,4-triazol-3-yl)-1H-indazol-3-yl)benzamide Synthesis of (I-14)
[0145]
[0146] 1. Synthesis of N-cyclopropyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide (compound 1-2)
[0147]
[0148] (1) Synthesis of 3-bromo-N-cyclopropylbenzamide (compound 9)
[0149] Take 3-bromobenzoic acid (500mg, 2.5mmol), HATU (1.43mmol, 3.75mmol) and DIEA (806mg, 6.25mmol), dissolve it in DMF, stir at room temperature for 15min, add cyclopropylamine (171mg, 3mmol), and react at room temperature for 3h ; The end of the reaction was detected by TLC, the reaction solution was diluted with water, extracted and dried with EA, and the target product (400mg, 67%) was obtained by silica gel column chromatography. ESI-MS m / z:240.0[M+H] + .
[0150] (2) N-cyclopropyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzamide (compound 1-2) synthesis
[0151] Take 3-bromo-N-cyclopropylbenzamide (400mg, 1.67mmol), biboronic acid pin...
Embodiment 3
[0157] Example 3: 3-(5-(4-isopropyl-4H-1,2,4-triazol-3-yl)-1H-indazol-3-yl)benzamide (compound I-20) Synthesis
[0158]
[0159] 1. Synthesis of 3-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyridine (compound 1-3)
[0160]
[0161] (1) Synthesis of 3-(3-bromophenyl)pyridine (compound 10)
[0162] Take 1,3-dibromobenzene (343mg, 1.46mmol), 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine ( 300mg, 1.46mmol), Pd (pph 3 ) 4 (168mg, 0.146mmol) and K 2 CO 3 (404mg, 2.92mmol), with Dioxane / H 2 O was dissolved at 5:1, reacted overnight at 80°C; TLC detected the end of the reaction, removed the insolubles by suction filtration, washed the filter cake with EA, diluted the filtrate with water, extracted the reaction solution with water, and extracted with EA, dried and spin-dried the EA, and obtained by silica gel column chromatography Target product (300 mg, 89%). ESI-MS m / z:234.0[M+H] + .
[0163] (2) 3-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com