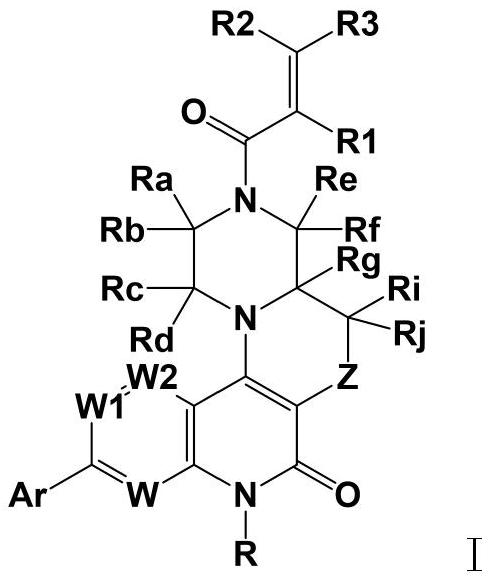
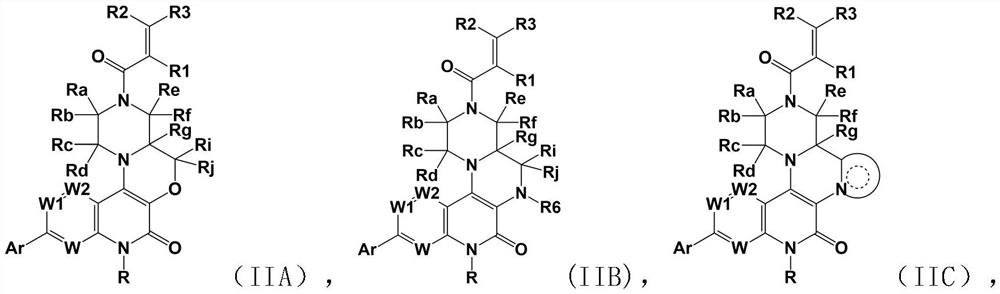
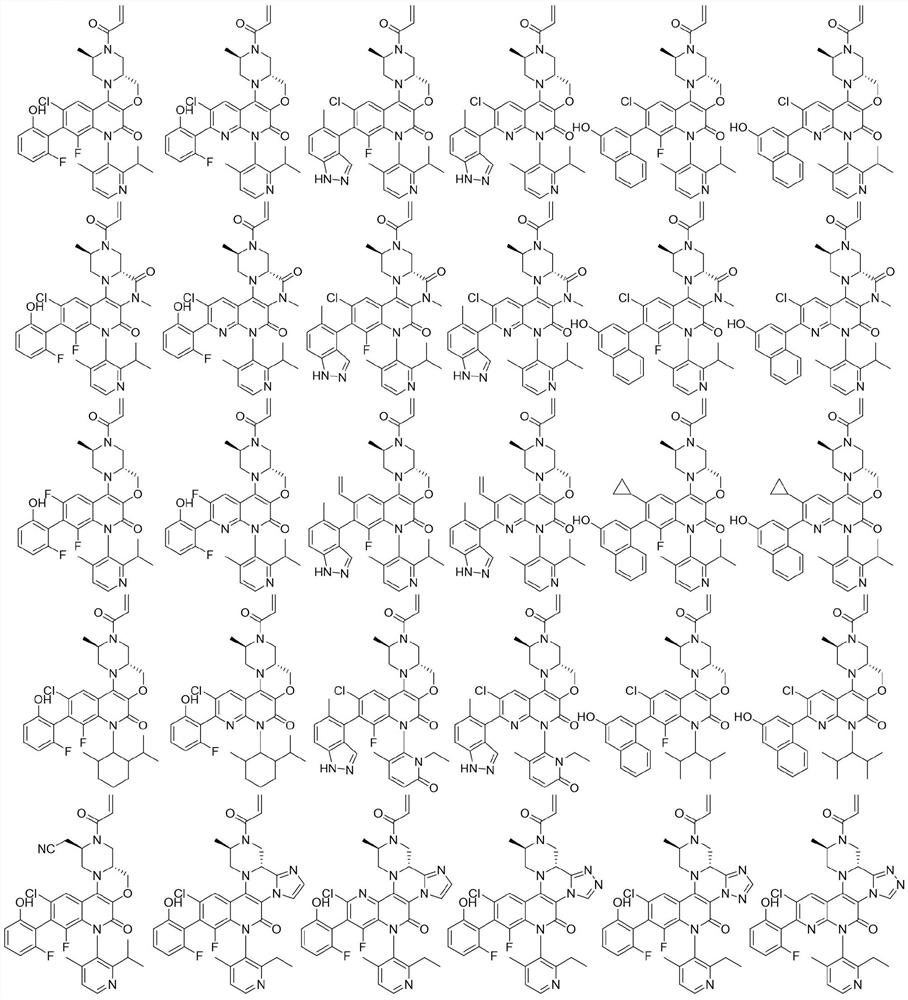
Fused ring pyridone compound, preparation method and application
A technology of condensed ring pyridone and compound, applied in the field of medicinal chemistry, can solve the problems such as the failure to successfully develop targeted drugs and the small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0110] Embodiment General preparation method two
[0111]
[0112] Step 1: Dissolve the fused ring pyridone intermediate (1eq.) in anhydrous tetrahydrofuran, add DIPEA (1.6eq.) and piperazine intermediate (1.5eq.) in sequence, and heat to reflux for 18 hours under nitrogen protection . The reaction was complete as monitored by TLC, cooled to room temperature, and concentrated under reduced pressure. The residue was separated by adding water and dichloromethane. The aqueous phase was extracted three times with dichloromethane. The extract was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The target product was obtained by chromatography separation and purification, and its structure was confirmed by NMR and mass spectrometry.
[0113] The second step: dissolve the above-mentioned first step product (1eq.) in anhydrous glacial acetic acid, slowly add reduced iron powder (3eq.), and heat to 80 degrees under nitrogen protection and stir for 0.5 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com