Hydrophilic anthracene fluorescent dye and synthesis method thereof
A technology of fluorescent dyes and synthesis methods, applied in the field of hydrophilic anthracene fluorescent dyes and their synthesis, can solve the problems of reduced quantum efficiency, poor water solubility, etc., and achieve the effects of increased self-stability, easy storage, and widened selection range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
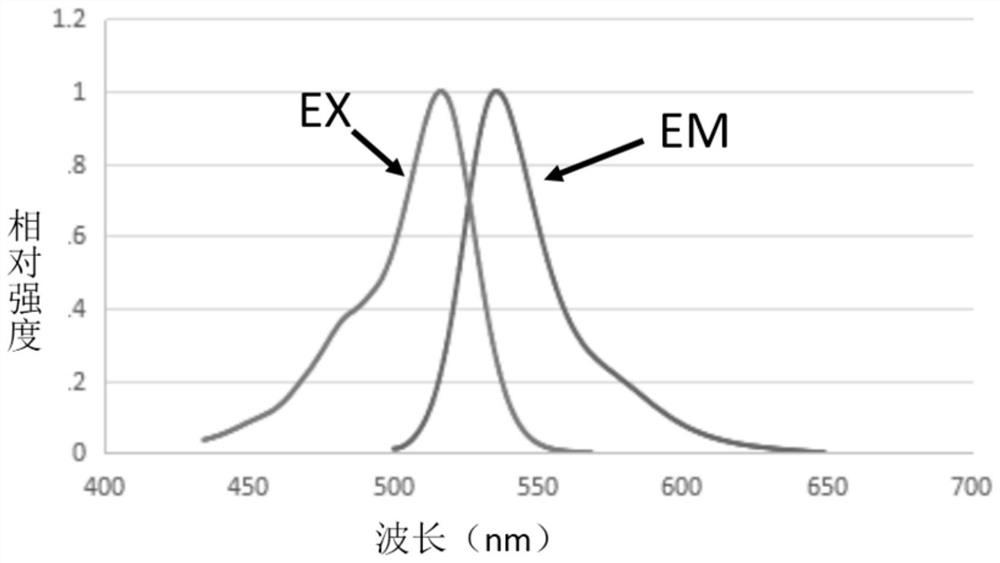
Image
Examples
Embodiment Construction
[0035] Exemplary embodiments of the present disclosure are described in more detail below. It should be understood that the present disclosure can be implemented in various forms and should not be limited by the embodiments set forth herein. Rather, these embodiments are provided for more thorough understanding of the present disclosure and to fully convey the scope of the present disclosure to those skilled in the art.
[0036] Unless otherwise specified, the words involved in the present invention are common words in this field.
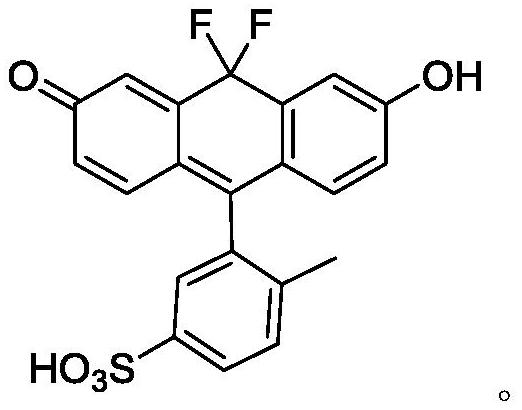
[0037] The anthracene compounds involved in the present invention refer to compounds having a triphenylcyclanthracene-like structure. This term is not a special term.
[0038] In the reaction involved in the present invention, there are other products in certain steps, but on the basis of the description of the present invention, those skilled in the art can easily obtain the target product. The specific structure of the by-product does not belo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com