Synthesis method and application of binuclear zinc complex
A technology of zinc complexes and synthesis methods, applied in the fields of zinc organic compounds, organic chemical methods, 2/12 group organic compounds without C-metal bonds, etc. , low cost, mild reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
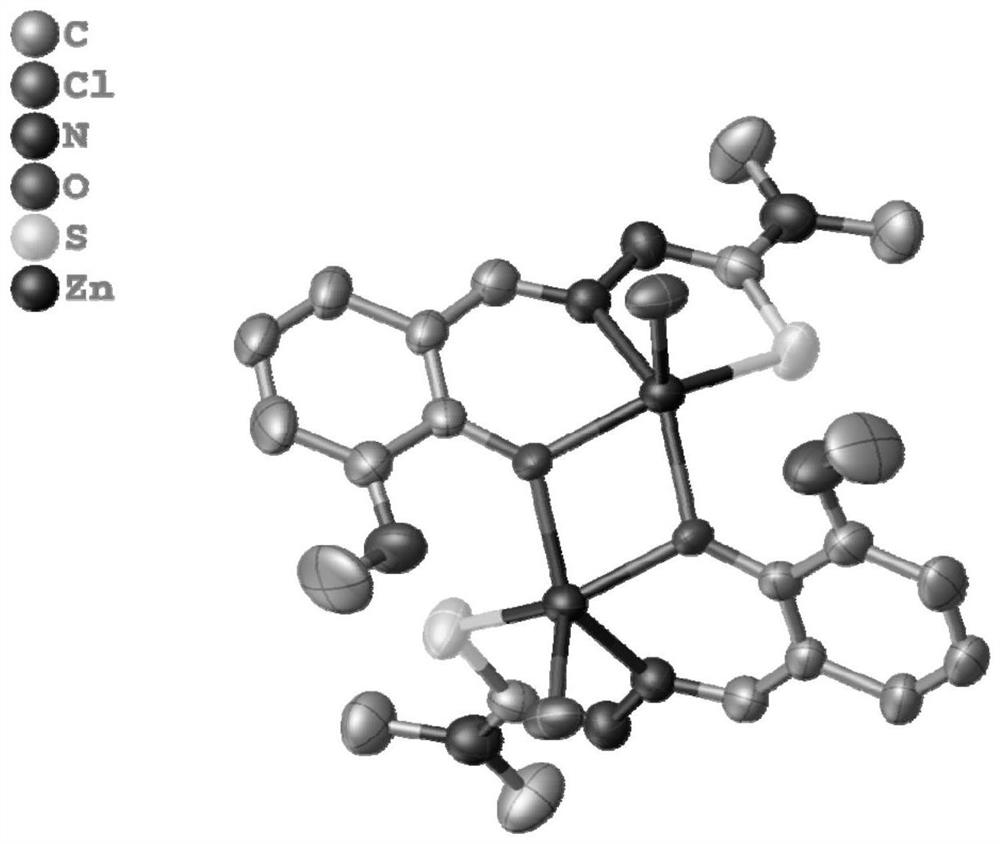
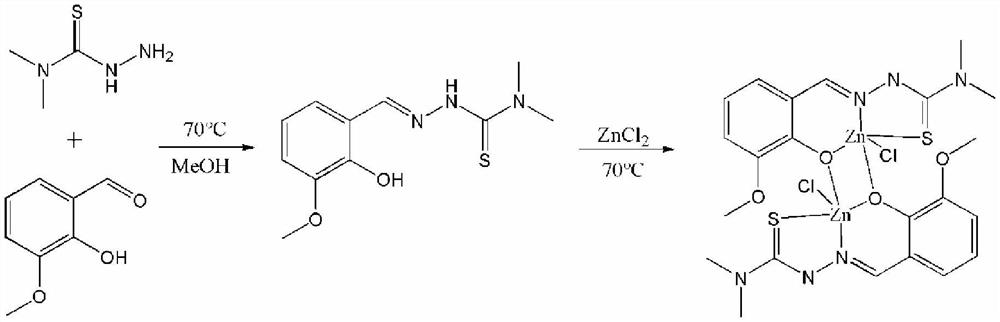
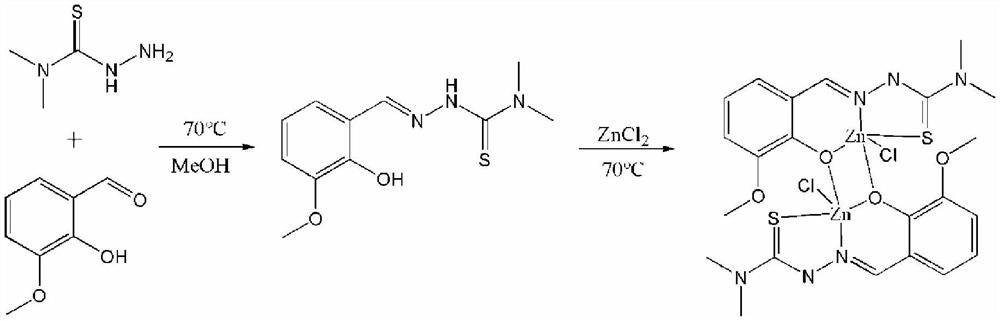
[0033] A method for synthesizing a binuclear zinc complex, that is, a method for synthesizing a zinc complex of o-vanillin condensed 4,4-dimethyl-3-thiosemicarbazide, the reaction route of which is as follows:
[0034]
[0035] Concrete reaction steps are as follows:
[0036] 1) Dissolve 5 mmol of o-vanillin and 5 mmol of 4,4-dimethyl-3-thiosemicarbazide in 50 mL of methanol, and stir until dissolved;
[0037] 2) The above mixed solution was refluxed and stirred at 70°C for 6 hours to obtain a light yellow solution;
[0038] 3) The yellow solution was rotary evaporated under reduced pressure to obtain a light yellow powder, washed three times with a small amount of saturated sodium bicarbonate solution, and dried to obtain the ligand. Yield: 91.1%, mass spectrometry, ESI+m / z: C 11 h 15 N 3 o 2 S, 254.09[M+H] + ;
[0039] 4) Weigh 0.1mmol of ligand and 0.1mmol of zinc chloride and dissolve in 20mL of methanol, and continue stirring under reflux for 4h;
[0040] 5) Aft...
Embodiment 2
[0047] A kind of synthetic method of binuclear zinc complex, namely a kind of synthetic method of o-vanillin condensed 4,4-dimethyl-3-thiosemicarbazide zinc complex, its synthetic method reaction route is the same as embodiment 1, specific reaction Proceed as follows:
[0048] 1) Dissolve 4mmol of o-vanillin and 4mmol of 4,4-dimethyl-3-thiosemicarbazide in 45mL of methanol, and stir until dissolved;
[0049] 2) The above mixed solution was refluxed and stirred at 65°C for 8 hours to obtain a light yellow solution;
[0050] 3) The yellow solution was rotary evaporated under reduced pressure to obtain a light yellow powder, washed three times with a small amount of saturated sodium bicarbonate solution, and dried to obtain the ligand. Yield: 91.0%, mass spectrometry, ESI+m / z: C 11 h 15 N 3 o 2 S, 254.09[M+H] + ;
[0051] 4) Weigh 0.08mmol of ligand and 0.12mmol of zinc chloride and dissolve in 18mL of methanol, and continue stirring under reflux for 3h;
[0052] 5) After ...
Embodiment 3
[0055] A kind of synthetic method of binuclear zinc complex, namely a kind of synthetic method of o-vanillin condensed 4,4-dimethyl-3-thiosemicarbazide zinc complex, its synthetic method reaction route is the same as embodiment 1, specific reaction Proceed as follows:
[0056] 1) Dissolve 6 mmol of o-vanillin and 4 mmol of 4,4-dimethyl-3-thiosemicarbazide in 55 mL of methanol, and stir until dissolved;
[0057] 2) The above mixed solution was refluxed and stirred at 75°C for 4 hours to obtain a light yellow solution;
[0058] 3) The yellow solution was rotary evaporated under reduced pressure to obtain a light yellow powder, washed three times with a small amount of saturated sodium bicarbonate solution, and dried to obtain the ligand. Yield: 90.3%, mass spectrometry, ESI+m / z: C 11 h 15 N 3 o 2 S, 254.09[M+H] + ;
[0059] 4) Weigh 0.12mmol of ligand and 0.08mmol of zinc chloride and dissolve in 22mL of methanol, and continue to reflux and stir for 5h;
[0060] 5) After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com