Reagent for in-vitro detection on mutation of SelS gene promoter and application of reagent in preparation of coronary heart disease screening kit
An in vitro detection and promoter technology, which is applied in the determination/testing of microorganisms, biochemical equipment and methods, etc., can solve the problems of inaccurate positioning of specific sites and fragments, difficulties in CAD prevention and treatment drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
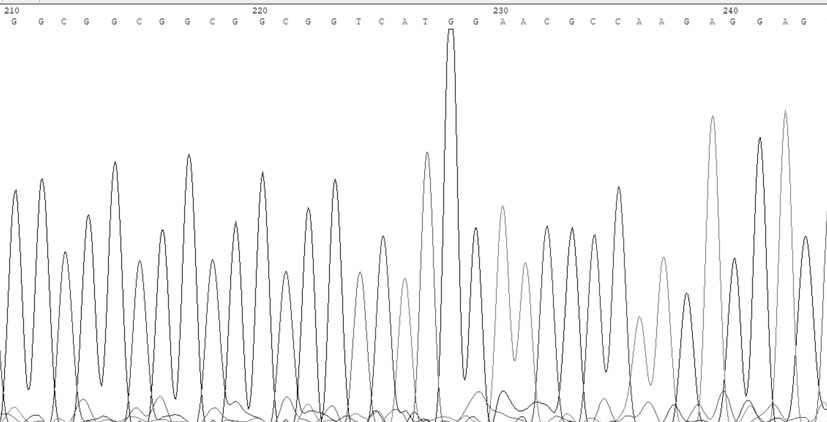
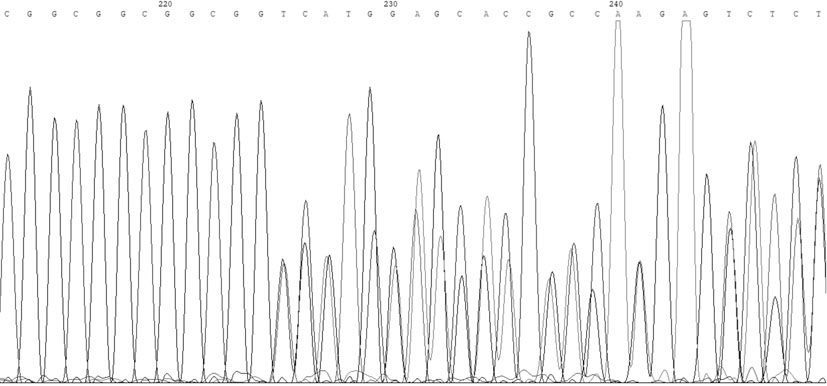
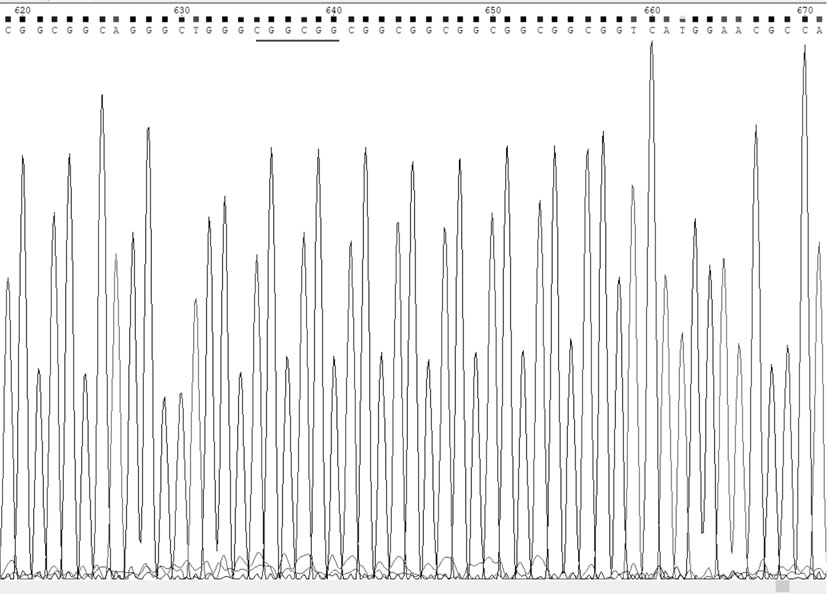
[0034] Example 1: A coronary heart disease susceptibility gene is a mutated SelS gene promoter, and a test sample containing the SelS gene can be obtained from the blood of the tester. When the SelS gene promoter is mutated, the 84th to 89th positions in the nucleotide sequence table (shown in SEQ ID NO: 1) are deleted.
Embodiment 2
[0035] Embodiment 2: A reagent or kit for detecting SelS gene promoter mutation in vitro, the kit includes primers, probes and amplification reagents.
[0036] The nucleotide sequences of the primers are as follows:
[0037] Upstream primer (the nucleotide sequence is shown in SEQ ID NO: 2 in the sequence listing): GAAATTCGGTAAGAAATCCGTAAC;
[0038] Downstream primer (the nucleotide sequence is shown in SEQ ID NO: 3 of the sequence listing): CGCAACGACTCACCCGTGG;
[0039] The nucleotide sequence of the probe is as follows:
[0040] SelS wild-type TP probe sequence (the nucleotide sequence is shown in SEQ ID NO: 4 in the sequence listing): CTGGGCGGCGGCGGCGGCGGCGGCGGCGGT;
[0041] SelS wild-type BM probe sequence (the nucleotide sequence is shown in SEQ ID NO: 5 in the sequence listing): ACCGCCGCCGCCGCCGCCGCCGCCGCCCAG;
[0042] SelS mutant TP probe sequence (the nucleotide sequence is shown in SEQ ID NO: 6 in the sequence listing): CTGGGCGGCGGCGGCGGCGGCGGT;
[0043] SelS muta...
Embodiment 3
[0045] Example 3: Screening of SelS gene promoter mutation sites
[0046] In this study, 48 patients with coronary heart disease who were hospitalized in the Cardiology Department of the First Affiliated Hospital of Xinjiang Medical University from January 2016 to December 2017 were selected as the sequencing objects.
[0047] Inclusion criteria: CAD group: at least one coronary artery stenosis ≥50% confirmed by percutaneous coronary angiography in our hospital and no other heart disease. Control group: People without any heart disease in the population chronic disease survey in Xinjiang from 2016 to 2019 were selected, and the two groups signed an informed consent form before being included in the study.
[0048] Exclusion criteria: CAD group: patients with incomplete clinical data and one of the following diseases were excluded. (eg: aortic dissection, rheumatic heart disease, congenital heart disease, multiple organ failure, and those with mental disorders who cannot coope...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com