A rapid detection kit for circulating tumor cells
A detection kit and technology for tumor cells, applied in the field of rapid detection kits for circulating tumor cells, to achieve the effects of easy mastery by clinical personnel, objective results, and reduced burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Kit composition and usage method.
[0035] (1) The components of the kit mainly include:
[0036] ① Oncoquick tumor cell density gradient separation solution 4ml (Geriner, Germany);
[0037] ② 10 porous membrane separation tubes with a volume of 15mL (Wuhan Haijili Biotechnology Co., Ltd.);
[0038] ③ 10 centrifuge tubes with a volume of 5 mL (Eppendorf, Germany);
[0039] ④ 488 Mouse Anti-CK7 / CK8 (BD, USA);
[0040] ⑤ BB515MouseAnti-Human CD326 antibody (BD, USA);
[0041] ⑥ Anti-Human CD325 (N-Cadherin) PE (eBioscience, USA).
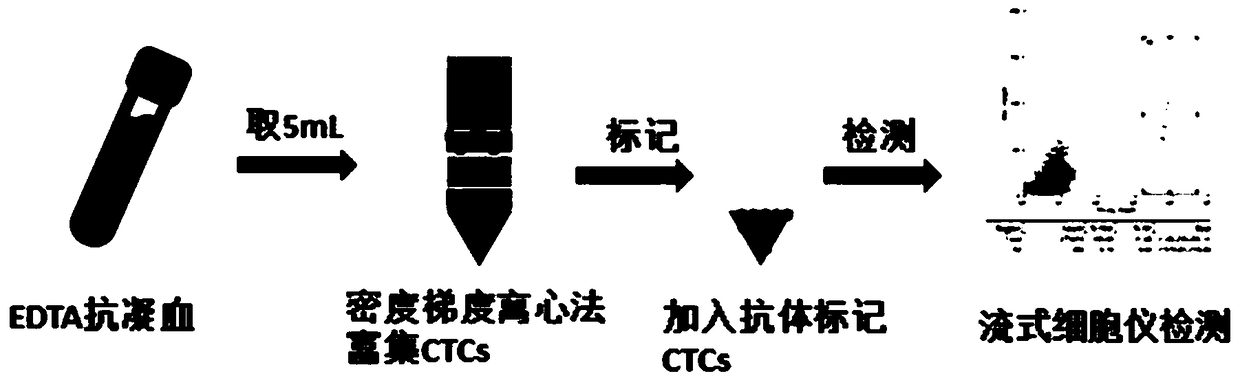
[0042] (2) The specific detection process is as follows (see figure 1 , kit detection process flow chart):
[0043] ①Blood collection 5mL whole blood EDTA anticoagulant (stored at 4 degrees and pre-cooled; test within 24 hours)
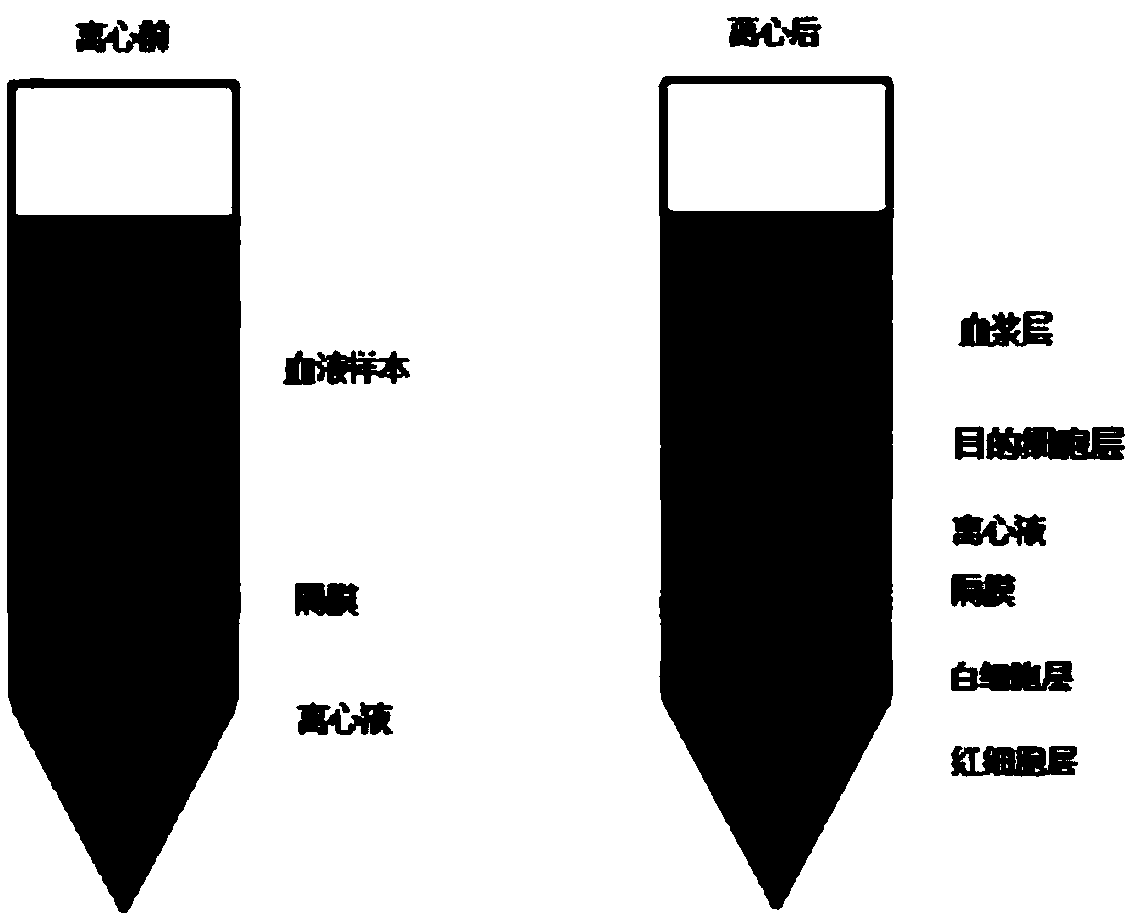
[0044] ② Take 5 mL of peripheral blood from tumor patients and add it into a centrifuge tube with separation solution, and centrifuge at 1200 g for 25 minutes. After centrifugation, the peripher...
Embodiment 2
[0049] Example 2: Detection and analysis of simulated samples of healthy human peripheral blood mixed with tumor cell lines
[0050] In this example, the peripheral blood of healthy people was mixed with gradiently diluted MCF-7 cells, and it was used as a simulated sample for detection and analysis, so as to evaluate the tumor cell enrichment efficiency of the kit and the recovery rate of the kit, etc. Performance, detailed as follows:
[0051] 1. Sample preparation
[0052] The MCF-7 cells in the culture dish were digested and made into a single cell suspension, counted with a red blood cell counting plate, and diluted to an appropriate concentration with PBS. Add different concentrations of MCF-7 cells to 5 mL of healthy human peripheral blood anticoagulated with EDTA, and the concentrations are 800 / tube, 400 / tube, 100 / tube, 20 / tube, 5 / tube, 0 pcs / tube. 5 sets of repetitions were set for each concentration.
[0053] 2. Sample detection
[0054] (1) Gently invert the simu...
Embodiment 3
[0066] Example 3: Extraction and Analysis of Individual Samples of Circulating Tumor Cell DNA
[0067] 1. Sample preparation
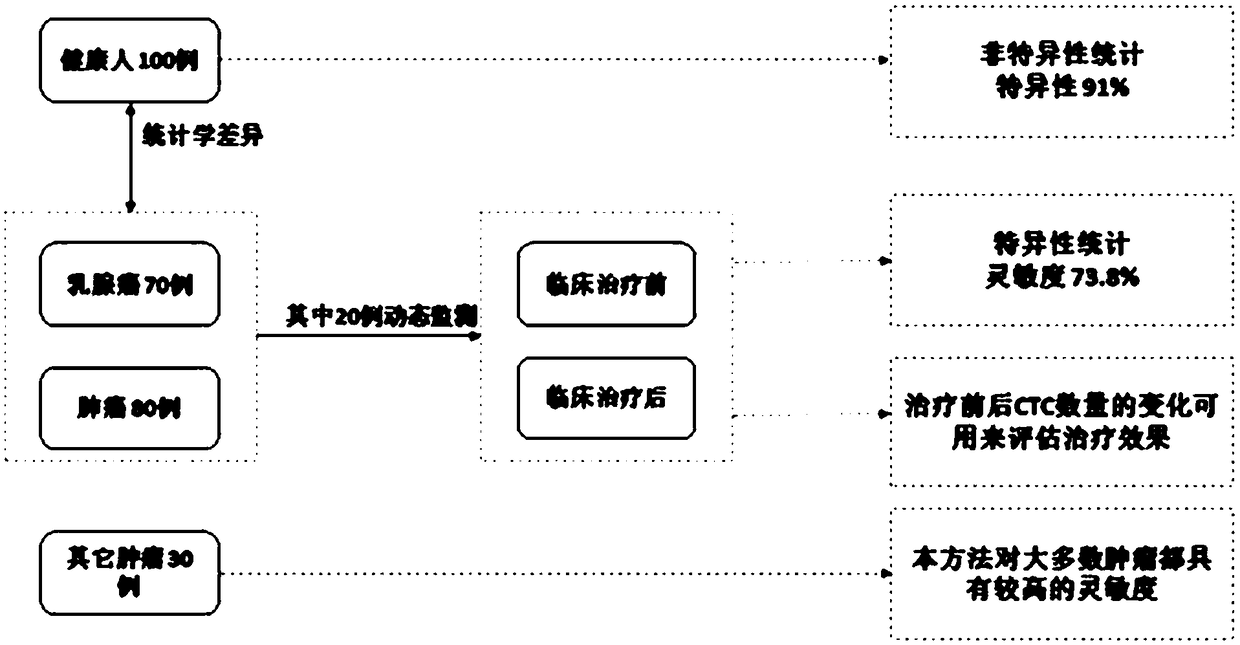
[0068] The peripheral blood of 100 healthy people, the peripheral blood of 79 patients with breast cancer, the peripheral blood of 80 patients with lung cancer, and the peripheral blood of 30 patients with other primary tumors were selected as test samples (attached image 3 ).
[0069] 2. Sample detection:
[0070] The experimental process is as in Example 2.
[0071] 3. Test results: see attached Figure 4 .
[0072] The median value of normal population detection is: 1.8;
[0073] The median detection value of the tumor patient population is: 7.9;
[0074] There was a significant difference between the two groups (P<0.0001).
[0075] Table 2. Positive coincidence rate table of tumor patient population
[0076]
[0077] When 5 is selected as the cutoff value, it is obtained from the ROC curve model: the sensitivity is 73.8%, and the specif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com