Crystal of benzoxazole derivative
A crystal and benzo technology, applied in the crystal field of benzoxazole derivatives, can solve the problems that have not yet been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
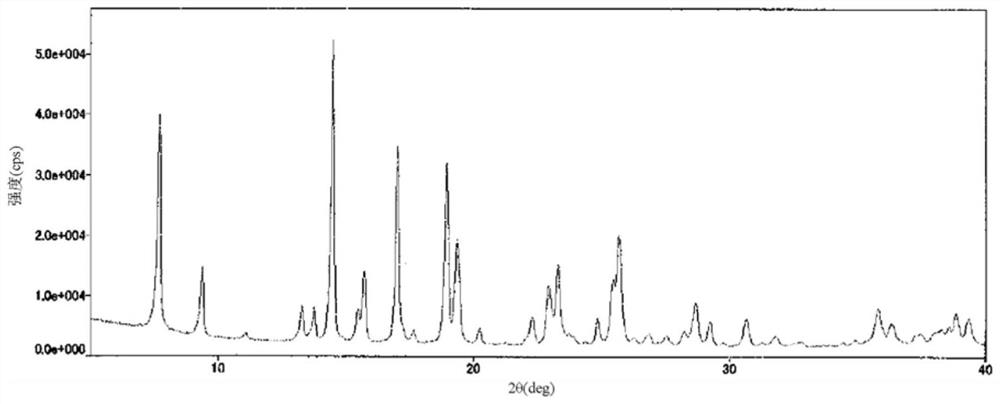
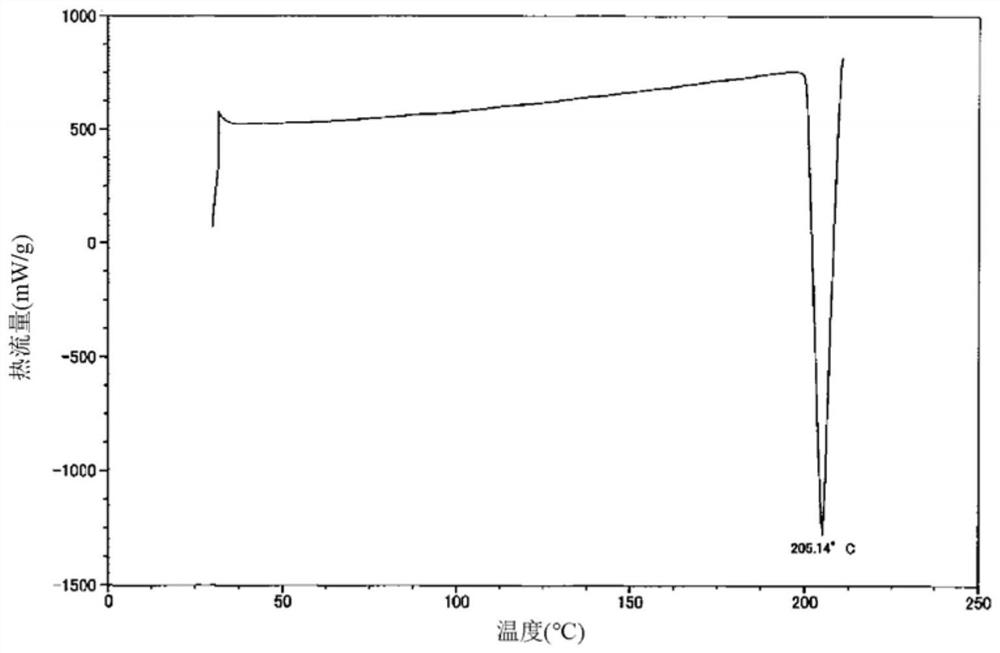
[0065]21.9 mL of a mixed solvent of ethyl acetate and 2-propanol (1:1, v / v) was added to the solid (219 mg) of the compound represented by formula (1) obtained in Reference Synthesis Example 1, and heated to 60 ℃ to make it dissolved. The solution was concentrated to dryness, and 4.38 mL of a mixed solvent of ethyl acetate and 2-propanol (1:1, v / v) was added to the obtained residue, and the mixture was stirred at 45°C for 1 hour. After the temperature was brought to room temperature, 21.9 mL of hexane was added and stirred for 1 hour. After filtration, it was washed with hexane and dried to obtain 194 mg of type I crystals. The obtained type I crystals have diffraction angles (2θ±0.2°) based on X-ray powder diffraction, 7.7°, 9.4°, 13.2°, 13.7°, 14.5°, 15.7°, 17.0°, 18.9°, 19.4° , 22.9°, 23.3°, 25.7°, 28.7°, 35.7° show characteristic peaks. The X-ray powder diffraction pattern of type I crystal is shown infigure 1 . A differential scanning calorimetry (DSC) analysis of the obtained ...
Embodiment 2
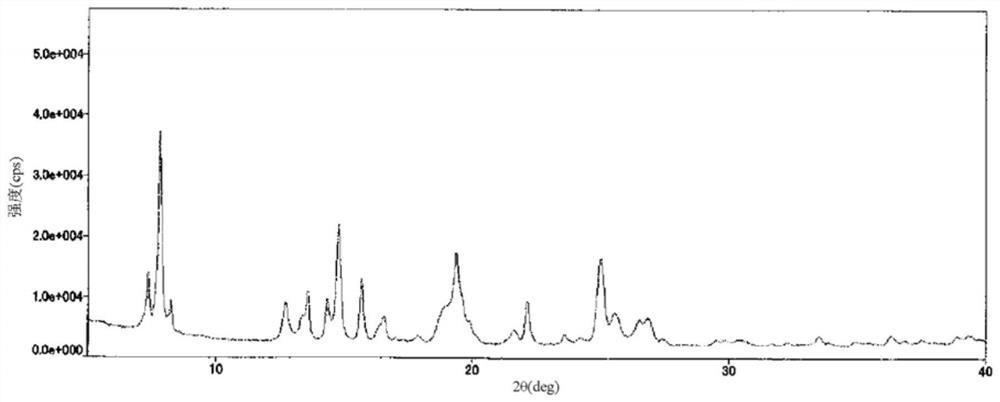
[0067]To 584 mg of type I crystals obtained in Example 1, 50 mL of methanol was added and dissolved. Concentration under reduced pressure was performed so that the weight became about 1 / 6, and the resulting homogeneous solution was cooled to 0°C and stirred for 10 minutes, thereby depositing a solid. After concentration and drying, 35 mL of a mixed solvent of hexane and ethyl acetate (2:1, v / v) was added to the residue, and the mixture was stirred at room temperature for 30 minutes. After filtration, it was washed with a mixed solvent of hexane and ethyl acetate (2:1, v / v) and dried to obtain 566 mg of type II crystals. The obtained type II crystals are the diffraction angles (2θ±0.2°) based on X-ray powder diffraction, 7.4°, 7.8°, 8.2°, 12.7°, 13.6°, 14.3°, 14.7°, 15.7°, 16.5° , 19.3°, 22.1°, 25.0°, 25.6° show characteristic peaks. The X-ray powder diffraction pattern of type II crystal is shown infigure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com