Phosphoramidite and its preparation method and use
A technology of phosphoramidite and diisopropyl phosphoramidite, which is applied in the field of phosphoramidite and its preparation, can solve the problems of cumbersome synthesis steps, high price and difficult separation and purification of photodegradable reagents, and achieve good results. Effects of photoresponsiveness, good market value, and high photoresponse efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] Wherein the etherification reaction in the preparation method of the present invention is under the above reaction conditions, so that 4,4'-dimethoxytriphenylchloromethane can be preferentially combined with benzyl alcohol in 5-hydroxyl-2-nitrobenzyl alcohol The middle hydroxyl group reacts while avoiding the etherification of the phenolic hydroxyl groups on the remaining benzene rings. This operation is carried out at 0°C, which can also avoid the possible decomposition of 4,4'-dimethoxytriphenyl at higher temperatures, which helps to simplify subsequent purification steps and reduce operating costs.
[0039] In an optional embodiment: the purification treatment specifically includes the following steps: taking the crude product and dissolving it in ethyl acetate, washing with water, drying, filtering, and rotary evaporation to remove the organic solvent, and then dissolving the product in ethyl acetate , added dropwise into cooled n-hexane, filtered and dried to obtai...
Embodiment 1
[0058] A preparation method of phosphoramidite, comprising the following steps:
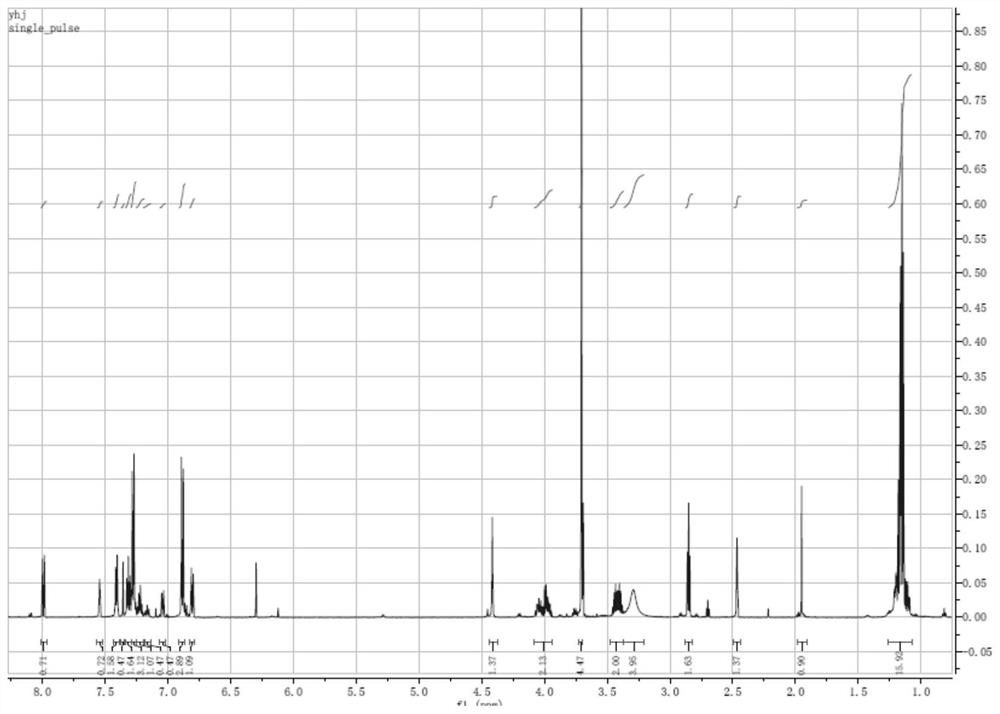
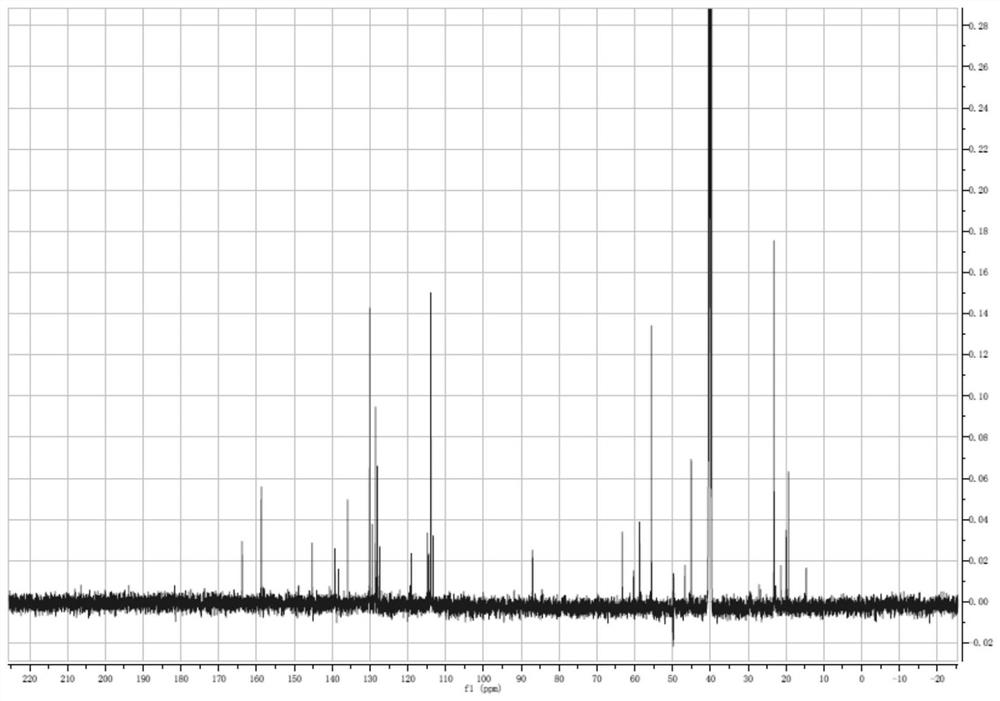
[0059] Step 1: Preparation of 5-hydroxy-2-nitrobenzyl-(4,4'-dimethoxytriphenyl) ether: Dissolve 1.7 g of 5-hydroxy-2-nitrobenzyl alcohol in 100 ml of Add dropwise to 30 ml of anhydrous pyridine dissolved in 4.0 g of 4,4'-dimethoxytriphenylchloromethane under ice-bath stirring, and stir the reaction solution at 0°C after the addition is complete for 1 hour, returned to room temperature and stirred overnight; after the reaction was completed, the organic phase was rotary evaporated, and the crude product was dissolved in 30 ml of ethyl acetate, washed twice with 50 ml of water, and the organic phase was dried with anhydrous sodium sulfate; filtered After rotary evaporation to remove the organic solvent, the obtained dark yellow oily liquid was dissolved in 5 ml of ethyl acetate and added dropwise to 100 ml of n-hexane cooled to 0°C to obtain a yellow precipitate, which was filtered and dried in vac...
Embodiment 2
[0068] A method for preparing deoxyribonucleic acid containing a photosensitive unit, comprising the following steps:
[0069] Step 1: Take 600mg of 2-nitrobenzyl-(4,4'-dimethoxytriphenyl)ether-5-(2-O-cyanoethyl-N,N-dimethoxy) prepared in Example 1 Isopropyl phosphoramidite) was dissolved in 10 milliliters of anhydrous acetonitrile, and transferred to the reagent bottle labeled "5" of the ABI3400 DNA synthesizer under nitrogen protection, defined as X, and the DNA sequence was input, using the synthesis mode of DMT retention, Perform synthesis on the order of 1 μmol;
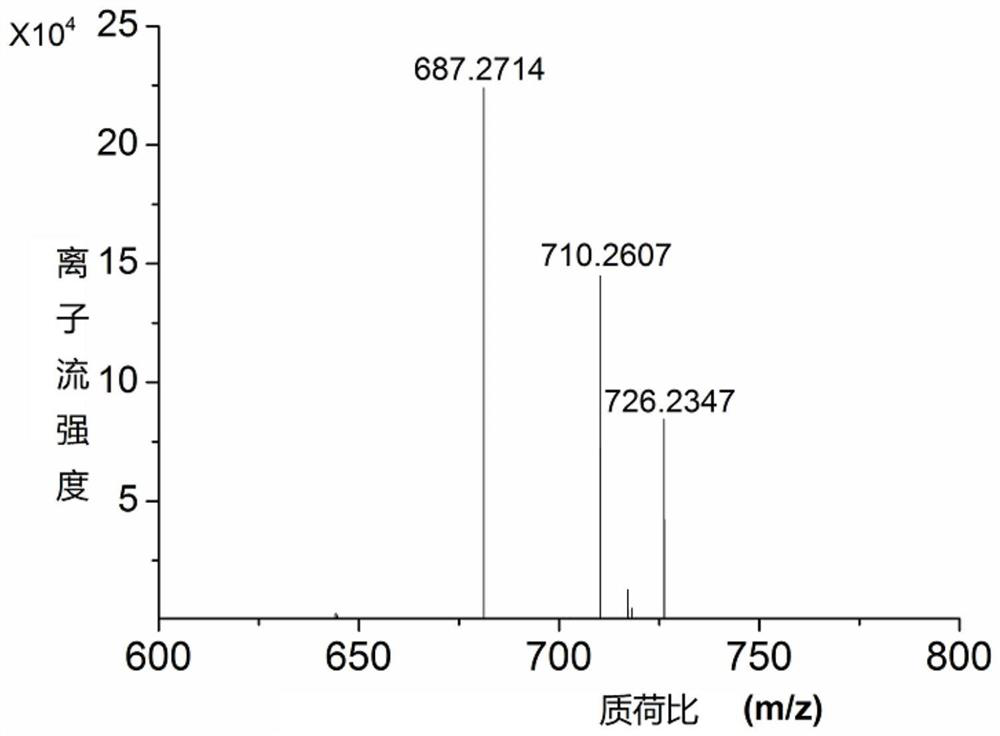
[0070] Step 2: After the synthesis is completed, take out the solid-phase carrier, disperse it in 1 ml of 23% ammonia water, heat it in an airtight manner at 55°C for 2 hours, centrifuge to remove the insoluble matter, and concentrate the solution to obtain the crude product; pass the nucleic acid containing the photosensitive unit of the target product through High-performance liquid phase separation, and stru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com