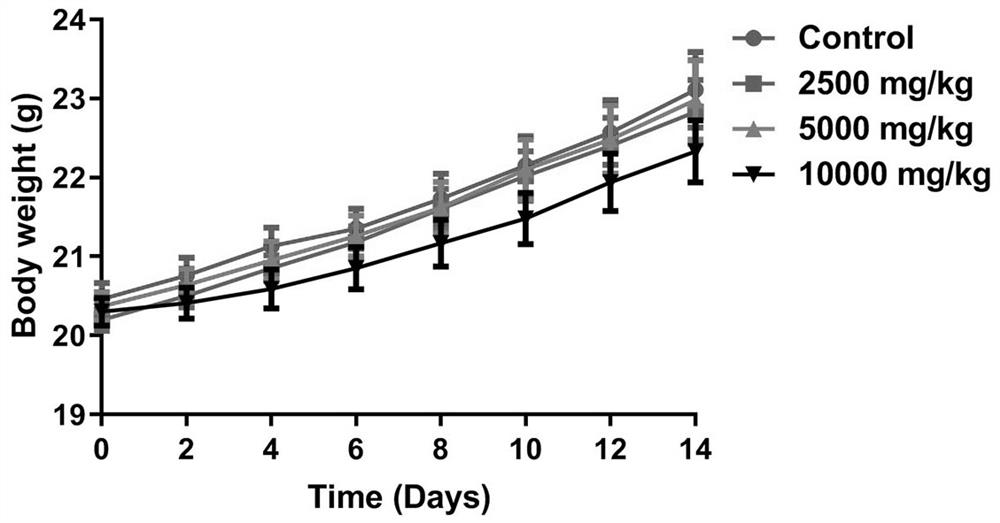
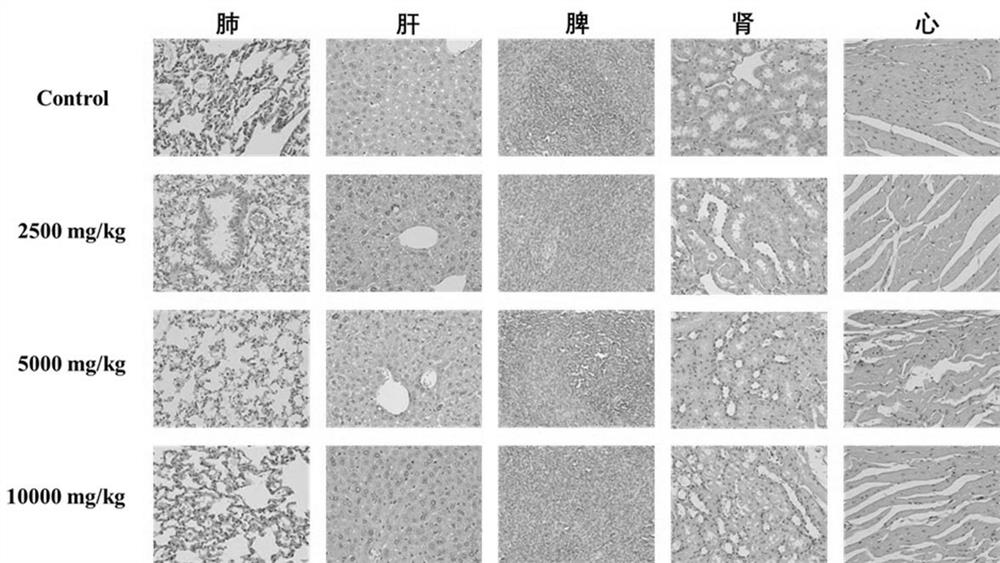
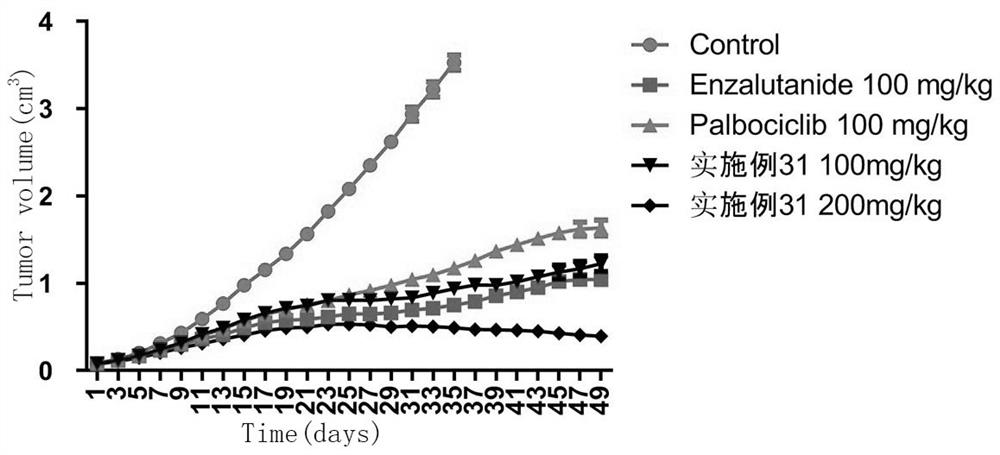
CDK6/DYRK2 double-target inhibitor as well as preparation method and application thereof
A CH2, C1-C8 technology, applied in the field of CDK6/DYRK2 dual-target inhibitors and their preparation, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] (6-((4-(Benzothiazol-6-yl)-5-fluoropyrimidin-2-yl)amine)pyridin-3-yl)(4-ethylpiperazin-1-yl)methanone (I -1) Synthesis:
[0149]
[0150] Compounds 6-(2-chloro-5-fluoropyrimidin-4-yl)benzothiazole (133 mg, 0.5 mmol) and 6-aminopyridin-3-yl)(4-ethylpiperazin-1-yl)methyl Ketone (141 mg, 0.6 mmol) was dissolved in dioxane (5 mL), then Pd was added 2 (dba) 3 (23 mg, 0.025 mmol), Xantphos (58 mg, 0.1 mmol), cesium carbonate (326 mg, 1.0 mmol), replace argon three times, heat to 100°C, and react for 12 h. Cooling filtration concentration column chromatography (DCM~DCM / MeOH=10:1) obtains compound (6-((4-(benzothiazol-6-yl)-5-fluoropyrimidin-2-yl)amine)pyridine-3 -yl)(4-ethylpiperazin-1-yl)methanone (88 mg, 38% yield). 1 H NMR (300 MHz, CDCl 3 ) δ 9.68 (s, 1H), 9.15 (s, 1H), 8.76– 8.75 (m, 1H), 8.61 – 8.58 (m, 2H), 8.52 (dd, J = 8.7, 0.9 Hz, 1H), 8.33 –8.26 (m, 2H), 7.86 (dd, J = 8.7, 2.3 Hz, 1H), 3.80 – 3.61 (m, 4H), 2.51 –2.44 (m, 6H), 1.12 (t, J = 7.2 Hz, 3H). ...
Embodiment 2
[0152] 4-(benzothiazol-6-yl)-N-(5-((4-ethylpiperazin-1-yl)methyl)pyridin-2-yl)-5-fluoropyrimidin-2-amine (I -2) Synthesis:
[0153]
[0154] Referring to the synthetic method of compound (I-1), the yield was 43%. 1 H NMR (300 MHz, CDCl3) δ 9.15 (s,1H), 9.11 (s, 1H), 8.78 – 8.76 (m, 1H), 8.54 (d, J = 3.5 Hz, 1H), 8.41 (d, J = 8.6 Hz, 1H), 8.35 – 8.26 (m, 3H), 7.73 (dd, J = 8.6, 2.3 Hz, 1H), 3.52 (s,2H), 2.58 – 2.47 (m, 10H), 1.14 (t, J = 7.2 Hz, 3H).
Embodiment 3
[0156] tert-Butyl 4-((6-((4-(benzothiazol-6-yl)-5-fluoropyrimidin-2-yl)amine)pyridin-3-yl)methyl)piperazine-1-carboxylic acid Synthesis of ester (I-3):
[0157]
[0158] Referring to the synthetic method of compound (I-1), the yield was 52%. 1 H NMR (400 MHz, CDCl 3 ) δ 9.14 (s,1H), 8.83 (s, 1H), 8.77 (d, J = 1.5 Hz, 1H), 8.52 (d, J = 3.5 Hz, 1H), 8.41(d, J = 8.6 Hz, 1H), 8.34 – 8.27 (m, 3H), 7.75 – 7.73 (m, 1H), 3.50 (s, 2H),3.45 – 3.43 (m, J = 5.2 Hz, 4H), 2.43 – 2.40 (m, 4H), 1.46 (s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com